INTRODUCTION
Upper endoscopy facilitates the detection and treatment of gastrointestinal (GI) disease. Antispasmodic agents such as hyoscine butylbromide, atropine, glucagon, cimetropium bromide, and L-menthol are often administered prior to GI endoscopy to inhibit peristalsis and improve visualization.1,2 However, these agents must be administered intravenously or intramuscularly and may cause adverse effects such as dry mouth, urinary retention, temporary impairment of visual accommodation, palpitations, anaphylactic shock, and hyperglycemia.3,4,5 Cimetropium bromide (Algiron; Boehringer Ingelheim GmbH, Ingelheim, Germany) is used particularly frequently in South Korea. Cimetropium bromide can cause pain and preprocedural anxiety due to its administration by intravenous or intramuscular injection, the preparation of which is time-consuming.6,7
Phloroglucin (Flospan; Daehwa Pharmaceutical, Seoul, Korea), administered orally, was expected to reduce pain and discomfort more effectively than intravenous or intramuscular injections of other antispasmodic agents. However, few studies of its usefulness as an endoscopic premedication have been performed.
In this study, we examined the efficacy of oral phloroglucin for the suppression of peristalsis, its impact on patient compliance, and any associated complications, and compared it with the intravenous or intramuscular administration of cimetropium bromide.
MATERIALS AND METHODS
This was a randomized, investigator-blind, prospective comparative study. From August 2012 through May 2013, we enrolled 174 patients who visited the Ewha Womans University Mokdong Hospital. Eligible patients were aged 18 years or older and scheduled to be examined by esophagogastroduodenoscopy. Patients with a history of upper GI surgery, GI bleeding, pregnancy, or contraindications for anticholinergic agents (glaucoma, myasthenia gravis, and urinary obstruction) were excluded from the study. Written informed consent was obtained from all subjects before enrollment. This study was approved by the Ewha Womans University's Ethics Committee.
Patients were randomized into two groups according to the following medications administered prior to upper endoscopy: oral phloroglucin (group A) and cimetropium bromide (group B). All endoscopic procedures were performed by a single experienced endoscopists who was blinded to the patients' group assignments. We evaluated total procedure times (from insertion to removal), total number of peristalsis events (stomach and duodenal motility numbers, counted at the antrum and duodenal second portion for 30 seconds each), and patient responses to questionnaires assessing tolerance and adverse events during the procedure (mouth dryness, nausea, vomiting, dizziness, headache, and abdominal pain). The degree of peristalsis was assessed using visibility scores (range, 0 to 2) at the antrum and duodenal second portion (0, no peristalsis; 1, slight peristalsis but no obscured visibility; 2, severe peristalsis with obscured visibility).
RESULTS
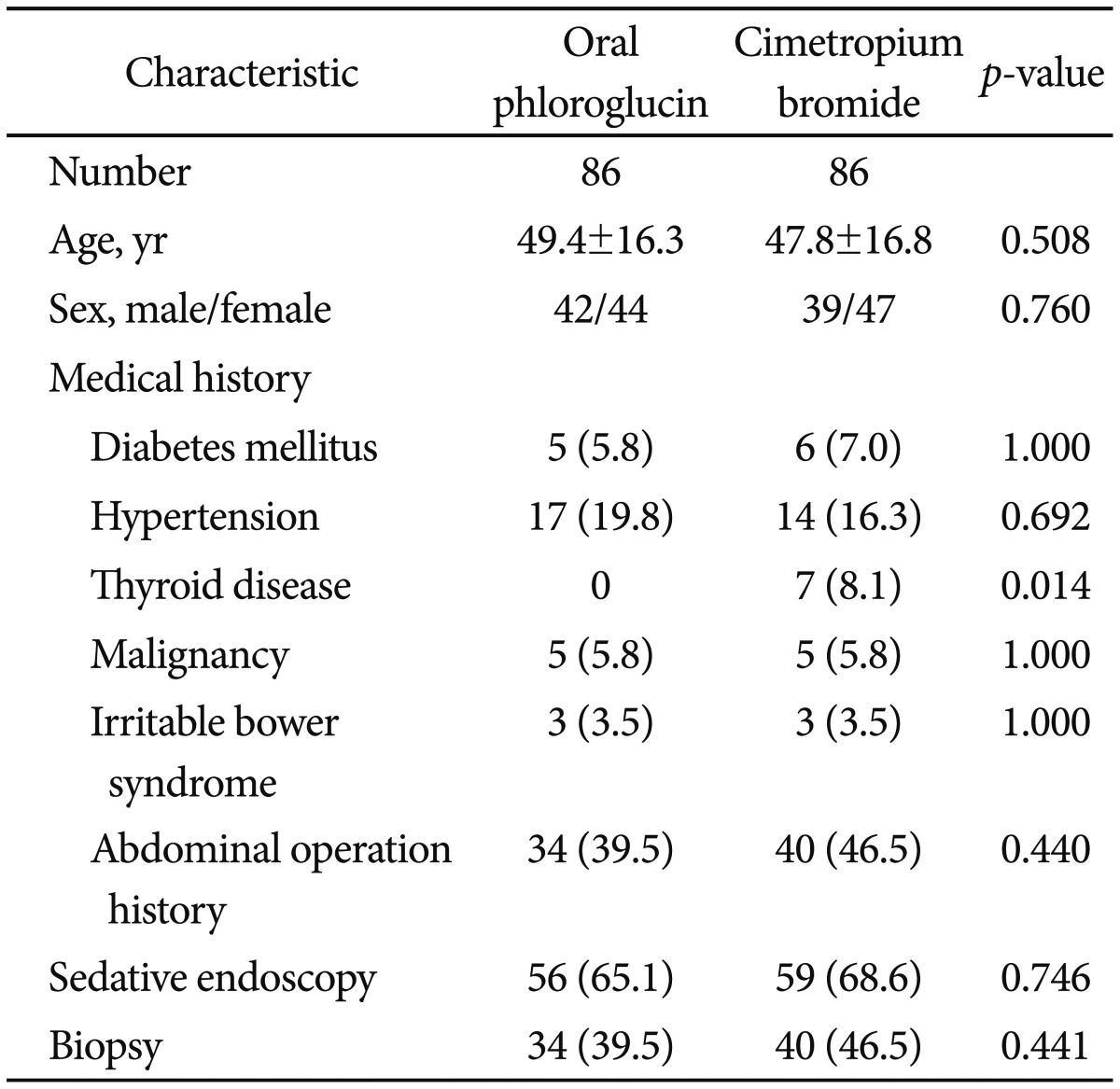
Among the 174 patients enrolled in the study, two were excluded because of severe duodenal stenosis. The remaining 172 patients were randomized into two groups according to medication administered prior to upper endoscopy, namely oral phloroglucin (group A, n=86) or cimetropium bromide (group B, n=86). The demographic and other baseline characteristics of included patients are shown in Table 1. There was no statistically significant difference between the two groups regarding age, sex, medical history (with the exception of thyroid disease), and the proportion of patients taking sedatives for endoscopy.
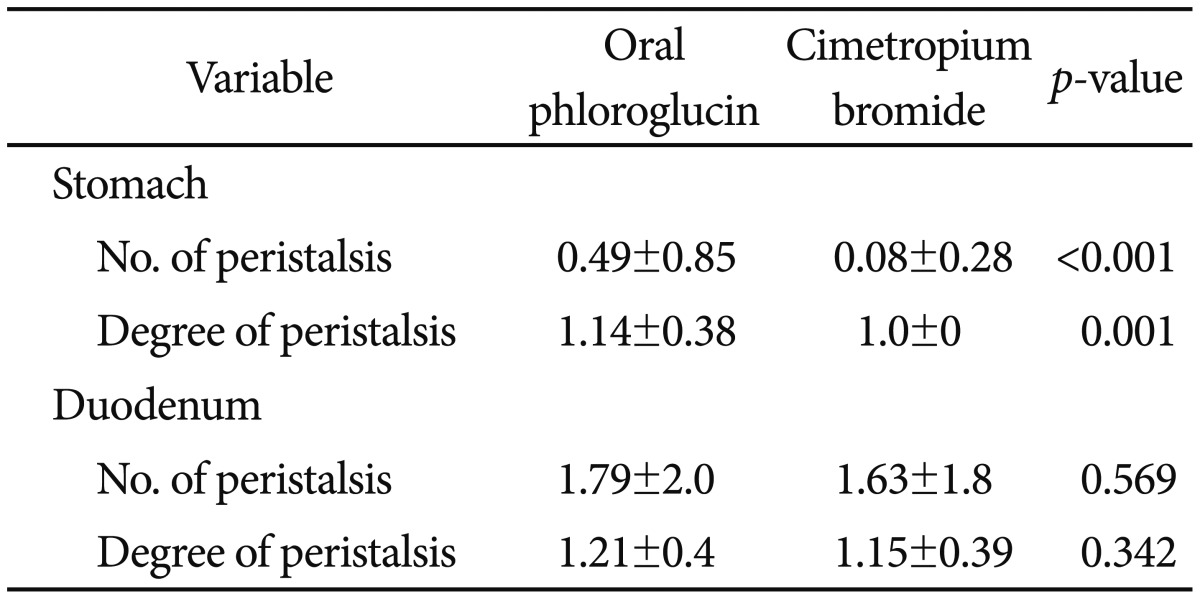
The incidence and degree of peristalsis in each group is presented in Table 2. A significantly higher number of gastric peristalsis events was seen in group A (0.49┬▒0.85 vs. 0.08┬▒0.28, p<0.001), but the number of events was fewer than one in both groups, and the difference was not clinically significant. The degree of peristalsis of the stomach was significantly lower in group B (1.14┬▒0.38 vs. 1.00, p=0.001), but both groups had visibility scores of approximately 1, with a clinically insignificant difference. No significant between-group difference was found for the number of duodenal peristalsis events (1.79┬▒2.0 vs. 1.63┬▒1.8, p=0.569). The degree of peristalsis of the duodenum did not differ significantly between the groups (1.21┬▒0.4 vs. 1.15┬▒0.39, p=0.342). There was no significant difference between the groups in total procedure time (5.28┬▒2.07 minutes vs. 5.10┬▒1.94 minutes, p=0.563). The incidence and degree of peristalsis and procedure time were not different between patients who received a biopsy and those who did not (Table 3).
Tolerance of endoscopy was not significantly different between the two groups, and the same number of patients tolerated the procedure well in both groups (group A, n=75, 87.2%; group B, n=75, 87.2%). No serious adverse events occurred in the course of the study. The incidence of adverse effects is presented in Table 4. The incidence of dry mouth was significantly higher with cimetropium bromide than with phloroglucin (50% vs. 15.1%, p<0.001). No significant between-group differences were noted for the incidence of other adverse events such as nausea, vomiting, dizziness, headache, dysuria, and abdominal pain.
DISCUSSION
Cimetropium bromide (Algiron) is often used before GI endoscopy to inhibit peristalsis and improve visualization, and is particularly popular in South Korea. However, cimetropium bromide causes pain and preprocedural anxiety due to its administration by intravenous or intramuscular injection, the preparation of which is time-consuming.
This study showed that oral phloroglucin is somewhat inferior to cimetropium bromide in the suppression of gastric peristalsis, but the difference was not clinically significant because the number of peristalsis events was less than one in both groups, and the degree of peristalsis was approximately grade 1 in both groups. In this study, we demonstrated that oral phloroglucin is not inferior to cimetropium bromide in the inhibition of peristalsis during endoscopy. In addition, endoscopic examination using oral phloroglucin was associated with similar procedure times, tolerance of endoscopy, and adverse events profiles. Furthermore, phloroglucin is superior to cimetropium bromide with respect to the incidence of dry mouth. An important advantage of oral phloroglucin is its ease of administration, effective suppression of peristalsis during endoscopy, and reduction in the incidence of dry mouth. Our findings suggest that oral phloroglucin can be used for the suppression of gastroduodenal peristalsis during upper endoscopy.
Our study had some limitations. We did not examine the effect of phloroglucin during endoscopic procedures such as endoscopic mucosal resection, endoscopic submucosal dissection, endoscopic retrograde cholangiopancreatography, and colonoscopy. Further studies are needed to examine the effects of oral phloroglucin during a variety of endoscopic therapeutic procedures and colonoscopy.
In conclusion, oral phloroglucin can be used as an antispasmodic agent during upper GI endoscopy with similar antispasmodic efficacy and fewer adverse effects when compared with cimetropium bromide.