See commentary "The Usefulness of New-Generation Capsule Endoscopy in Patients with Portal Hypertensive Enteropathy " in Volume 51 on page 505 AbstractBackground/AimsTo evaluate patients with portal hypertension (PH) of varied etiologies for portal hypertensive enteropathy (PHE) using the PillCam SB3 capsule endoscopy (CE) system.
MethodsConsecutive patients with PH presenting with unexplained anemia and/or occult gastrointestinal bleeding were evaluated using the PillCam SB3 CE system. Abnormal findings were categorized as vascular or non-vascular. The patients with ongoing bleeding caused by PHE were treated. The correlation of the CE scores of PHE with the clinical, laboratory, and endoscopic features was determined.
ResultsOf the 43 patients included in the study, 41 (95.3%) showed PHE findings. These included varices (67.4%), red spots (60.5%), erythema (44.2%), villous edema (46.5%), telangiectasia (16.3%), and polyps (16.3%). The CE scores varied from 0 to 8 (meanĀ±standard deviation, 4.09Ā±1.8). Five patients (11.6%) showed evidence of ongoing or recent bleeding due to PHE. Three of these five patients underwent endotherapy, and one patient underwent radiological coil placement.
INTRODUCTIONEsophageal and gastric varices are the most important consequences of portal hypertension (PH) [1]. The existence and importance of other mucosal changes in the stomach and colon, often referred to as portal congestive gastropathy (PCG) [2] and colopathy [3-6], respectively, have also been documented. Endoscopic evaluation of the changes in the small intestines is challenging. Some studies have reported the occurrence of histological changes in the small intestines [7-11]. Capsule endoscopy (CE) and device-assisted enteroscopy are recent advances that allow the evaluation of abnormalities in the small intestinal mucosa. A few recent case reports and case series have reported CE findings in PH [12-23]. However, such reports are scarce, and no study has reported the use of new third-generation CEs employing the recently launched PillCam SB3 CE system, which allows better and more comprehensive evaluation of the small intestines [24-26]. Thus, in the present study, we aimed to evaluate the small intestines of patients with PH using the PillCam SB3 CE system.
MATERIALS AND METHODSThis study was conducted from October 2016 to January 2017 and included 43 consecutive patients with PH who presented at our institute with unexplained anemia or occult gastrointestinal bleeding. This study was approved by our Institutional Review Board.
All patients were diagnosed with PH based on the clinical findings of ultrasonography and upper gastrointestinal endoscopy. The etiology of PH was evaluated on the basis of the patient history (alcoholism, drug use, etc.), liver function test findings, serological test findings (Hepatitis B surface antigen and antibodies against Hepatitis C virus), and other blood parameters (S-ceruloplasmin levels and autoimmune marker positivity) when required. The type of anemia was determined on the basis of red blood cells morphology as well as serum iron content, iron-binding capacity, and ferritin and vitamin B12 levels when required. Fecal occult blood test was performed if necessary. Upper gastrointestinal endoscopy and colonoscopy were performed to detect ongoing bleeding as well as its source if present. In addition, the patients suspected of having hypersplenism underwent splenic sequestration study. The exclusion criteria were as follows: age of <12 years, evidence of congestive cardiac failure, and history of non-steroidal anti-inflammatory drug use in the last 1 month.
The following patient data were recorded: age, sex, etiology of PH (alcohol, virus, etc.), platelet count, liver function test results, Child-Pugh class, and model for end stage liver disease (MELD) score. The presence and grade of esophageal varices (small: <5 mm, large: >5 mm), PH gastropathy (mild/severe) [2], and gastric varices were evaluated using upper gastrointestinal endoscopy. The presence of colonic varices and colopathy was assessed using colonoscopy. History of recent gastrointestinal bleeding was also recorded. History of endotherapy in the form of band ligation for esophageal varices and glue injection for gastric varices was also noted.
The PillCam SB3 CE system (Given Imaging, Yoqneam, Israel) along with the Rapid Reader Software v8.0 was used for CE. All patients were asked to fast overnight prior to the procedure, and 2 L of polyethylene glycol was used to prepare their small intestines for CE. Progression of capsule was confirmed via real time visualization. If the capsule remained in the stomach 90 minutes after ingestion, 25 mg of levosulpiride was injected intravenously.
The CE findings were evaluated by two experienced observers with an experience of >1,000 CE assessments. In case of any difference in opinion, the observers had discussions to reach an agreement. The CE findings were characterized as normal or abnormal. The presence of blood or ongoing bleeding observed during CE was also noted. The abnormal findings were characterized as vascular (red spots, telangiectasia, or varices) and non-vascular or inflammatory (villous edema, erythema, or polyps). The number and sites (proximal/middle/distal) of these abnormalities were also noted. These endoscopic findings have been previously characterized and defined [27]. A scoring system (modified from that described by Abdelaal et al. [18] and Kodama et al. [28]) was used to score the abnormal findings. A score of 1 each was given for the presence of any of the six findings if they were solitary and a score of 2 each if they were numerous. Thus, the score could range from 0 (normal CE) to 12 (presence of all six types of lesion, two or more in number). Esophageal and gastric findings revealed by CE, including the presence of esophageal varices, gastric varices, and PCG, were also recorded. The patients with ongoing bleeding in the small intestines were treated with endotherapy or radiological intervention.
The endoscopic scoring of portal hypertensive enteropathy (PHE) based on the CE findings was correlated with age and sex of the patients, history of gastrointestinal bleeding and endotherapy, hemoglobin level, platelet count, international normalized ratio (INR), albumin level, bilirubin level, alanine aminotransferase level, Child-Pugh class, MELD score, presence and grade of esophageal varices, presence of gastric varices, portal hypertensive gastropathy, colopathy, and colonic varices. Continuous data with normal distribution were presented as means and standard deviations (SDs). Qualitative variables were compared using the chi-square test or Fischerās exact test. Quantitative variables were compared between the groups using the Studentās t-test. P<0.05 was considered to indicate statistically significant differences.
RESULTSIn total, 43 patients fulfilled the inclusion criteria during the study period. Two patients had extrahepatic PH, whereas the 41 remaining patients had cirrhosis of varying etiologies (alcohol: eight patients, viral: 12 patients, and etc.: 21 patients). The demographic data and clinical and laboratory profiles of these patients are presented in Table 1.
No patient showed any adverse effect after CE. Small bowel preparation was excellent, and capsule retention was not observed in any patient. Only two patients required intravenous injection of levosulpiride to accelerate the capsule movement. The CE findings were normal in only two patients; one or more abnormalities were observed in the 41 (95.3%) remaining patients. Table 2 presents the CE findings of the small intestines of all study patients. Flat, nodular, or elevated varices were the most common finding in 67.4% of the patients, followed by red spots (60.5%), erythema (44.2%), and villousedema (46.5%). Telangiectasia and small intestinal polyps were the least common findings (each in 16.3% of the patients). Figs. 1 and 2 present the endoscopic images of the vascular and non-vascular findings, respectively.
The presence of fresh blood (Fig. 3) suggestive of ongoing or recent bleeding in the small intestines was observed in five patients (11.6%). The exact cause of bleeding could be identified in four of these five patients: red spots in two patients, varix in one, and telangiectasia in one. Three of these five patients had viral hepatitis B; one had hepatitis C; and one had alcoholic cirrhosis. The site of involvement was the proximal small intestines in 34 patients, middle small intestines in 22, and distal small intestines in 26. Thirty-two patients (78%) had more than one site of involvement. Of the nine patients with only one segment involved, eight patients had proximal segment involvement.
The CE scores varied from 0 to 8 (meanĀ±SD, 4.09Ā±1.8). Table 3A and B present the correlation of the CE scores with the various clinical, laboratory, and endoscopic parameters. Only three parameters, i.e., history of gastrointestinal bleeding, large size of esophageal varices, and non-alcoholic etiology of liver disease, were correlated with the endoscopic scores. No other parameter (i.e., age, sex, hemoglobin level, platelet count, INR, albumin level, bilirubin level, ascites, history of endotherapy, PCG, colopathy, gastric and colonic varices, Child-Pugh class, and MELD score) was correlated with the CE scores.
Upper gastrointestinal endoscopy revealed esophageal varices in 34 patients, gastric varices in six, and PCG in 33. CE could detect the lesion in 28 of the 34 patients with esophageal varices (82.4%), in none of the six patients with gastric varices (0%), and in 31 of the 33 patients with PCG (93.9%). The comparison of the CE features between the bleeding group (n=5) and the non-bleeding group (n=38) revealed a significant difference in the CE scores (meanĀ±SD, 4.26Ā±1.83 and 2.8Ā±0.84; P=0.033); all other parameters were similar between the two groups.
All patients with positive CE findings in the small intestines were administered or continued with Ī² blockers. Three patients underwent endotherapy with intravenous injection of a sclerosant for variceal bleeding (n=1) or argon plasma coagulation for non-variceal bleeding (n=2). One patient with jejunal bleeding was successfully treated with radiological coil placement.
DISCUSSIONPH often results in the development of varices at the sites of porto-systemic anastomosis [1]. It may also be associated with mucosal abnormalities at various sites in the gastrointestinal tract, collectively called portal hypertensive intestinal vasculopathy [2-6]. Owing to the poor accessibility of the small intestines, data on the endoscopic findings of the jejunum and ileum are limited [7-10]. The prevalence of PHE is high and varies from 40% to 96.8% [14-23], as shown in Table 4. This variation in the prevalence among studies may be attributable to the differences in their methodologies, particularly in the inclusion and exclusion criteria as well as in the definition of the various reported lesions. Moreover, the type of CE system used in the studies is also variable as most studies used first- or second-generation capsule systems [14-23].
The new third-generation CE system with the SB3 capsule and Rapid Reader Software v8.0 launched in 2016 is the most advanced system. It has an adaptive frame rate permitting the transmission of up to six frames per second according to the gut peristalsis rate and has better optics [26]. These capsules have approximately 12 hours of battery life, allowing complete views of the small intestines. Reportedly, the SB3 capsule has better sensitivity than previous-generation capsules [24,25]. Thus, the high prevalence of PHE based on the CE findings could be attributable to the ability of detection of the SB3 CE system.
The nomenclature used to describe various CE findings of PHE is variable among different studies. The literature describes PHE changes as mucosal edema, loss of vascularity, friability, hyperemia, red spots, angiodysplasia, pigmented black-brown spots, granularity, inflammatory polyps, and varices [21,23,27]. Interpretation of these lesions is subjective with considerable overlapping. We broadly divided the PHE changes into vascular and non-vascular/inflammatory lesions, and each type of lesions was further subdivided into three categories (Table 2). The overall relative prevalence of these lesions is consistent with reported data [27]. However, the higher prevalence of varices in our study could be attributable, at least partly, to the use of an advanced CE system, which allows better and more comprehensive evaluations.
CE revealed that the PHE changes were widely distributed in the entire length of the small intestines and often involved more than one site. However, the proximal small intestines were the most common site of involvement. Very few studies have evaluated the location of PHE [16-21]. Although Goulas et al. [21] noted changes in both the jejunum and ileum, Aoyama et al. [16] found the proximal and middle small intestines to be the common sites of involvement.
Although PHE is now an accepted condition because of CE studies, the exact significance of the lesions is not yet clear. PHE changes may be asymptomatic or could cause anemia [27]. Occasional cases of bleeding due to PHE, particularly from small intestinal varices, are well documented [11-13,29]. In the present study, blood was observed in the small intestines of 11.3% of the patients, suggesting bleeding due to PHE. In previous studies, active bleeding lesions were observed in up to 17.8% of patients, with bleeding occurring more commonly from vascular lesions than from non-vascular/inflammatory lesions [17,23,27].
Reports on the association of PHE with various demographic, clinical, and laboratory parameters are contradictory [18,23,27]. Most of the studies, including the present study, have found a direct correlation between PHE and the presence of large esophageal varices [18,23]. The study by Goulas et al. [21] and the present study found no association between PHE and advanced Child-Pugh class, which is contradictory to the findings of some other studies. Further, no association was found between the history of endotherapy for esophagogastric varices and PHE score on CE, which is consistent with the finding of a previous study [21]. The PHE score was higher in patients with a viral etiology of liver disease and in those with a history of gastrointestinal bleeding. Previous studies have also demonstrated a correlation of PHE with the computed tomography findings of PH [17] and high liver stiffness revealed by FibroScan [18].
Because of the limited studies on the clinical significance of PHE, no clear treatment is yet available. We used sclerotherapy to treat one patient with varix and argon plasma coagulation to treat two patients with inflammatory lesions. One patient with endoscopically inaccessible lesion was treated with angio-embolization. These therapeutic interventions have also been reported in previous case reports [30-32]. Polypectomy and argon plasma coagulation have been previously used for treating polypoidal lesions caused by PHE [27,33]. The use of transjugular portosystemic shunt in some cases has also been reported [34,35].
In the present study, we also evaluated the role of the SB3 CE system in assessing esophagogastric lesions in PH. This system was found to be highly sensitive in detecting PCG (93.9%) and fairly sensitive in detecting esophageal varices (82.4%). These results are somewhat better than those of previous-generation SB capsules, including esophageal capsules [36,37]. However, the system could not detect any of the six gastric varices probably because gastric varices are present as more localized lesions than PCG.
The strength of our study is the use of the SB3 CE system. However, the lack of a control group and follow-up data is a limitation. Further, we could not comment on the relationship between completeness (battery life) and prevalence. The significance of some of the detected lesions can be evaluated only with a longitudinal follow-up of these patients.
In conclusion, to the best of our knowledge, this is the first study on PHE that used a third-generation CE system. The system detected a high prevalence of PHE, particularly that of small intestinal varices. A small but definite proportion of the patients demonstrated bleeding due to PHE and could be successfully managed with endotherapy or radiological intervention.
Fig.Ā 1.Vascular changes seen on capsule endoscopy: (A) red spot, (B) telangiectasia, and (C) to (E) varices. 
Fig.Ā 2.Non-vascular (inflammatory) changes seen on capsule endoscopy: (A) villous edema, (B) and (C) erythema, and (D) and (E) polyp. 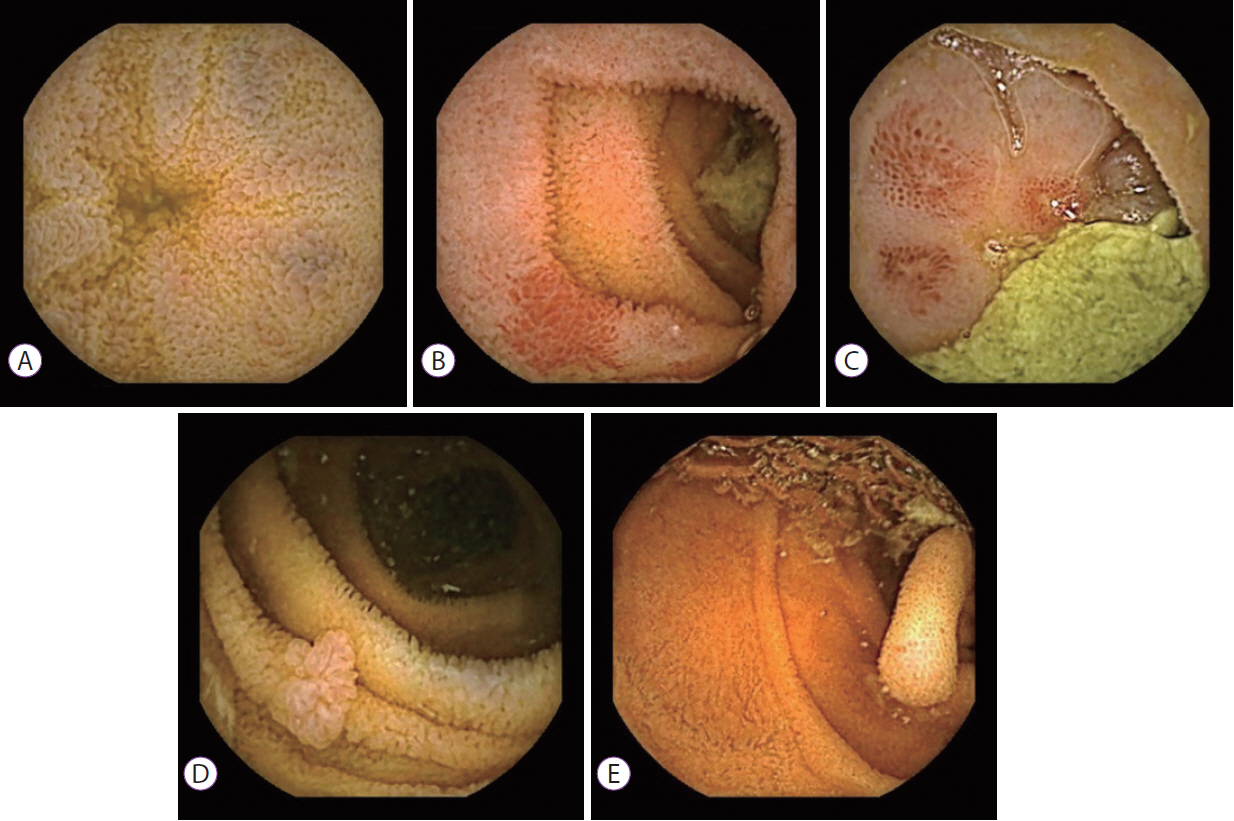
TableĀ 1.Demography and Clinical Profile of Patients (n=43)
TableĀ 2.Portal Hypertensive Enteropathy Changes at Capsule Endoscopy (n=41)
TableĀ 3A.Correlation of Endoscopy Score at Capsule Endoscopy with Clinical, Laboratory and Endoscopic Parameters
TableĀ 3B.Correlation of Endoscopy Score at Capsule Endoscopy with Clinical, Laboratory and Endoscopic Parameters
TableĀ 4.Studies Showing Prevalence of Portal Hypertensive Enteropathy as seen at Capsule Endoscopy
REFERENCES1. Bornman PC, Krige JE, Terblanche J. Management of oesophageal varices. Lancet 1994;343:1079ā1084.
2. McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut 1985;26:1226ā1232.
3. Ganguly S, Sarin SK, Bhatia V, Lahoti D. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology 1995;21:1226ā1231.
4. Goenka MK, Kochhar R, Nagi B, Mehta SK. Rectosigmoid varices and other mucosal changes in patients with portal hypertension. Am J Gastroenterol 1991;86:1185ā1189.
5. Chen LS, Lin HC, Lee FY, Hou MC, Lee SD. Portal hypertensive colopathy in patients with cirrhosis. Scand J Gastroenterol 1996;31:490ā494.
6. Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids, and portal hypertension. Lancet 1989;1:349ā352.
7. Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorphometric study of portal hypertensive enteropathy. Am J Clin Pathol 1997;108:652ā657.
8. Nagral AS, Joshi AS, Bhatia SJ, Abraham P, Mistry FP, Vora IM. Congestive jejunopathy in portal hypertension. Gut 1993;34:694ā697.
9. Viggiano TR, Gostout CJ. Portal hypertensive intestinal vasculopathy: a review of the clinical, endoscopic, and histopathologic features. Am J Gastroenterol 1992;87:944ā954.
10. Misra SP, Dwivedi M, Misra V, Gupta M. Ileal varices and portal hypertensive ileopathy in patients with cirrhosis and portal hypertension. Gastrointest Endosc 2004;60:778ā783.
11. Ohtani T, Kajiwara E, Suzuki N, et al. Ileal varices associated with recurrent bleeding in a patient with liver cirrhosis. J Gastroenterol 1999;34:264ā268.
12. Lewis P, Warren BF, Bartolo DC. Massive gastrointestinal haemorrhage due to ileal varices. Br J Surg 1990;77:1277ā1278.
13. Arst HF, Reynolds JD. Acute ileal variceal hemorrhage secondary to esophageal sclerotherapy. J Clin Gastroenterol 1986;8:603ā604.
14. Chandrasekar TS, Janakan GB, Chandrasekar VT, Kalamegam RY, Suriyanarayanan S, Sanjeevaraya PM. Spectrum of small-bowel mucosal abnormalities identified by capsule endoscopy in patients with portal hypertension of varied etiology. Indian J Gastroenterol 2017;36:32ā37.
15. Dabos KJ, Yung DE, Bartzis L, Hayes PC, Plevris JN, Koulaouzidis A. Small bowel capsule endoscopy and portal hypertensive enteropathy in cirrhotic patients: results from a tertiary referral centre. Ann Hepatol 2016;15:394ā401.
16. Aoyama T, Oka S, Aikata H, et al. Major predictors of portal hypertensive enteropathy in patients with liver cirrhosis. J Gastroenterol Hepatol 2015;30:124ā130.
17. Jeon SR, Kim JO, Kim JB, et al. Portal hypertensive enteropathy diagnosed by capsule endoscopy in cirrhotic patients: a nationwide multicenter study. Dig Dis Sci 2014;59:1036ā1041.
18. Abdelaal UM, Morita E, Nouda S, et al. Evaluation of portal hypertensive enteropathy by scoring with capsule endoscopy: is transient elastography of clinical impact? J Clin Biochem Nutr 2010;47:37ā44.
19. Akyuz F, Pinarbasi B, Ermis F, et al. Is portal hypertensive enteropathy an important additional cause of blood loss in portal hypertensive patients? Scand J Gastroenterol 2010;45:1497ā1502.
20. KovĆ”cs M, PĆ”k P, PĆ”k G, FehĆ©r J, RĆ”cz I. Small bowel alterations in portal hypertension: a capsule endoscopic study. Hepatogastroenterology 2009;56:1069ā1073.
21. Goulas S, Triantafyllidou K, Karagiannis S, et al. Capsule endoscopy in the investigation of patients with portal hypertension and anemia. Can J Gastroenterol 2008;22:469ā474.
22. Figueiredo P, Almeida N, LĆ©rias C, et al. Effect of portal hypertension in the small bowel: an endoscopic approach. Dig Dis Sci 2008;53:2144ā2150.
23. De Palma GD, Rega M, Masone S, et al. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc 2005;62:529ā534.
24. Monteiro S, de Castro FD, Carvalho PB, Moreira MJ, Rosa B, Cotter J. PillCamĀ® SB3 capsule: does the increased frame rate eliminate the risk of missing lesions? World J Gastroenterol 2016;22:3066ā3068.
25. Kunihara S, Oka S, Tanaka S, et al. Third-generation capsule endoscopy outperforms second-generation based on the detectability of esophageal varices. Gastroenterol Res Pract 2016;2016:9671327.
26. Eliakim R. Where do I see minimally invasive endoscopy in 2020: clock is ticking. Ann Transl Med 2017;5:202.
27. Mekaroonkamol P, Cohen R, Chawla S. Portal hypertensive enteropathy. World J Hepatol 2015;7:127ā138.
28. Kodama M, Uto H, Numata M, et al. Endoscopic characterization of the small bowel in patients with portal hypertension evaluated by double balloon endoscopy. J Gastroenterol 2008;43:589ā596.
29. HĆøjhus JH, Pedersen SA. Cirrhosis and bleeding ileal varices without previous intraabdominal surgery. A case report. Acta Chir Scand 1986;152:479ā480.
30. Varanasi RV, Fleisher AS, Darwin PE, King CE, Haluszka O. Colonoscopic sclerotherapy of ileal varices. Gastrointest Endosc 2000;52:109ā111.
31. Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology 1998;28:1154ā1158.
32. Koo SM, Jeong SW, Jang JY, et al. Jejunal variceal bleeding successfully treated with percutaneous coil embolization. J Korean Med Sci 2012;27:321ā324.
33. Lemmers A, Evrard S, Demetter P, et al. Gastrointestinal polypoid lesions: a poorly known endoscopic feature of portal hypertension. United European Gastroenterol J 2014;2:189ā196.
34. Guth E, Katz MD, Hanks SE, Teitelbaum GP, Ralls P, Korula J. Recurrent bleeding from ileal varices treated by transjugular intrahepatic portosystemic shunt: value of Doppler ultrasonography in diagnosis and follow-up. J Ultrasound Med 1996;15:67ā69.
35. Haskal ZJ, Scott M, Rubin RA, Cope C. Intestinal varices: treatment with the transjugular intrahepatic portosystemic shunt. Radiology 1994;191:183ā187.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
