INTRODUCTION
Obesity has become a pandemic, and its prevalence has increased significantly in recent decades. Obesity is a multifactorial disease caused by a combination of genetic, physiological, behavioral, sociocultural, and environmental factors. It shortens a personÔÇÖs life span and increases the risk of several chronic diseases, including type 2 diabetes mellitus (DM), hepatic disease, renal disease, cardiovascular disease, and malignancy [1,2].
Bariatric surgery is the most effective treatment for obesity [3]. In most countries, bariatric surgery is only indicated in patients with a body mass index (BMI) of >40 kg/m2 or >35 kg/m2 with severe comorbidities [4]. According to a recent study, the perioperative mortality rate following bariatric surgery is <1% in specialized centers [5]. However, <2% of the patients requiring treatment undergo bariatric surgery due to the following reasons: high operative risk, high cost, limited access, irreversible nature of the procedure, fear of re-gaining weight, and patientÔÇÖs preference [6]. Therefore, endoscopic bariatric treatment can be a suitable alternative for patients with obesity who are either not eligible for or do not prefer bariatric surgery. Recently, many endoscopic bariatric and metabolic therapies (EBMTs) have been developed and are generally classified into four categories: space-occupying, gastric restrictive, aspiration, and small bowel therapies. We aim to review the various EBMT techniques, focusing on non-balloon and non-gastroplasty devices, as the use of those devices is well described elsewhere in this issue of Clinical Endoscopy [7,8]. We will discuss the possible mechanisms of action, efficacy, and safety profiles of these EBMT methods.
ASPIRATION THERAPY
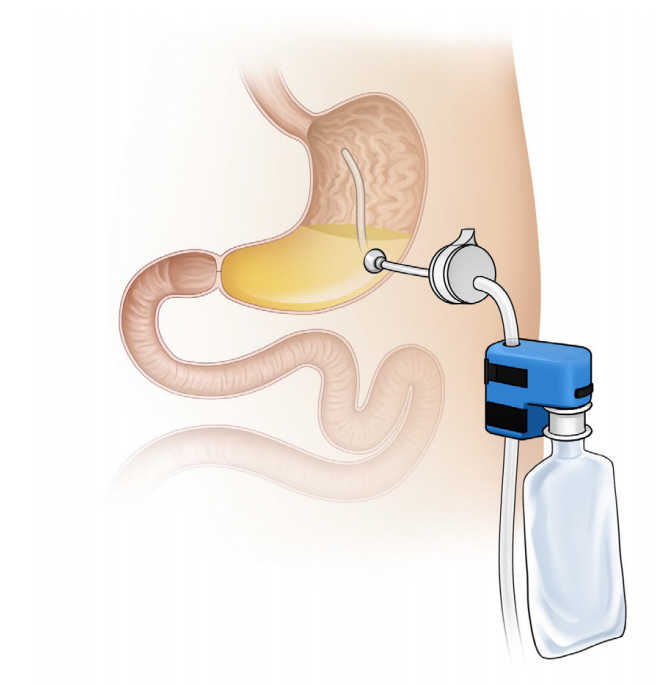
Aspiration therapy involves the aspiration of undigested food using a modified gastrostomy tube. Use of the AspireAssist device (Aspire Bariatrics, King of Prussia, PA, USA; Fig. 1) was approved in 2016 by the United States Food and Drug Administration (FDA) for adults aged 22 years with a BMI of 3555 kg/m2 who failed to achieve or maintain weight loss after receiving non-surgical weight loss therapies [9]. The device consists of an A-tube, a modified percutaneous endoscopic gastrostomy tube placed using the standard pull technique and a gravity flow director system through which the gastric contents are drained approximately 2030 minutes after ingestion. In aspiration therapy, weight loss is achieved through aspiration of calories and behavioral changes [10]. As the device drains the undigested food, the patients think about eating more carefully before meals. Furthermore, patients must chew the food properly and drink water during meals in order to aspirate food particles [11]. The device must be used after taking the daily meals (breakfast, lunch, and dinner). After 6 months, the valve of the device is likely to be blocked, thus preventing drainage. At this time, the patient needs to visit the clinic to change the port. This approach ensures patient follow-up and multidisciplinary team management.
Sullivan et al. first reported a pilot randomized controlled study comparing the percentage of total body weight loss (% TWL) and the percentage of excess body weight loss (% EWL) between patients receiving aspiration therapy (n=11, 10 completed the first year of the study) and those receiving lifestyle intervention only (n=7, 4 completed the first year of the study) [12]. They reported higher % TWL and % EWL among those who underwent aspiration therapy (%TWL: 18.6% ┬▒2.3% vs. 5.9% ┬▒5.0%, p=0.021; %EWL: 49% ┬▒7.7% vs. 14.9%┬▒12.2%, p=0.036) 1 year after receiving the aforementioned intervention. After 2 years, the aspiration therapy group (n=7) maintained a % TWL of 20.1%┬▒3.5% and a % EWL of 54.6%┬▒12.0%. In the largest multicenter randomized controlled study, 207 patients with obesity and a BMI of 35ÔÇô55 kg/m2 were randomly assigned to either the aspiration therapy plus lifestyle counseling group (n=137; mean BMI, 45.2┬▒5.1 kg/m2) or the lifestyle counseling only group (n=70; mean BMI, 40.9┬▒5.1 kg/m2) in a 2:1 ratio [13]. The aspiration therapy group achieved a mean % EWL of 31.5┬▒26.7% and a mean % TWL of 12.1 ┬▒9.6% at 52 weeks; the lifestyle counseling only group showed a mean % EWL of 9.8┬▒15.5% and a mean % TWL of 3.5┬▒6.0% (p<0.001). A multicenter post-market study conducted in five European clinics also confirmed the effectiveness of aspiration therapy [14]. In this study, the mean % TWL at 1 year was 18.2 ┬▒9.4% and was maintained at 19.2┬▒13.1% at 4 years. Furthermore, the metabolic parameters, such as glycated hemoglobin (HbA1c), triglyceride, and blood pressure levels, significantly decreased. In a meta-analysis including five studies on aspiration therapy, patients showed %TWL values of 17.8%, 18.3%, 19.1%, and 18.6% and %EWL values of 46.3%, 46.2%, 48.0%, and 48.7% at 1, 2, 3, and 4 years, respectively (p<0.0001 for all) [15]. An improvement was also observed in the metabolic parameters such as blood pressure, triglyceride, high-density lipoprotein, HbA1c, aspartate transaminase, and alanine transaminase levels.
The complications of aspiration therapy are usually due to the placement or maintenance of A-tube. The most commonly reported adverse events are peristomal granulation tissue (40.5%), abdominal pain within four weeks after A-tube insertion (37.8%), nausea or vomiting (17.1%), peristomal irritation (17.1%), intermittent abdominal pain (17.1%), and peristomal bacterial infection (13.5%) [13]. Although rare, serious adverse events such as peritonitis, severe abdominal pain, prepyloric ulcer induced by A-tube placement, A-tube malfunction, persistent gastrocutaneous fistula, and buried bumper syndrome have been reported [12-14].
DUODENAL-JEJUNAL BYPASS LINER
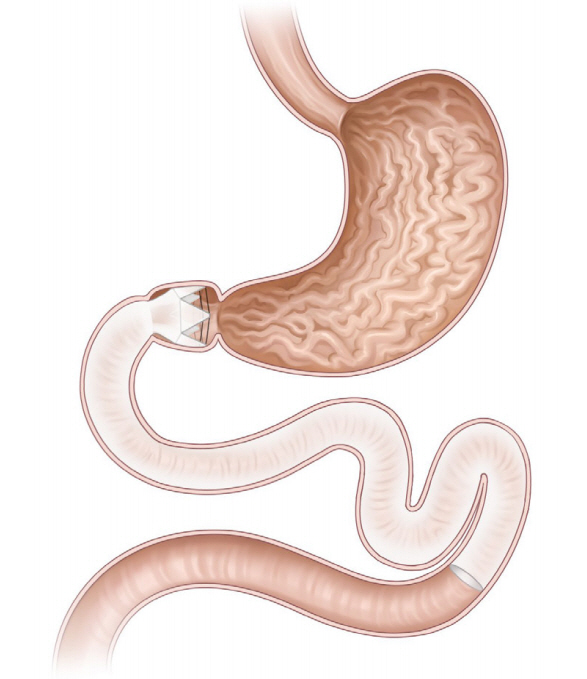
The duodenojejunal bypass liner (DJBL, EndoBarrier system; GI dynamics, Lexington, MA, USA; Fig. 2) is a 60-cmlong ultrathin, flexible, impermeable sleeve anchored in the duodenal bulb, and extends into the proximal jejunum. The device includes a liner, a delivery system, and a retrieval system. With the device implanted, ingested food particles from the stomach bypass the duodenum and go into the jejunum without mixing with the pancreatic juices and digestive enzymes, which pass through the space between the liner and the intestinal wall. The linear bypass portion is similar to that in Roux-en-Y gastric surgery. Although the exact mechanism of action of DJBL remains unknown, the possible mechanisms include the incretin effect, gut microbiota alteration, and bile flow modulation [16,17].
The efficacy of DJBL has been reported in several previous randomized controlled trials [18-23]. The largest randomized controlled trial was conducted in 77 patients with obesity and type 2 DM [22]. The DJBL was removed after 6 months. The % EWL values were 32.0% in the DJBL group vs. 16.4% in the control group (p<0.05) at 6 months and 19.8% in the DJBL group vs. 11.7% in the control group (p<0.05) at 12 months. A previous meta-analysis of four randomized controlled trials reported a significant decrease in mean body weight and %EWL of 5.1 kg (95% confidence interval [CI], 7.3 to 3.0; n [4 trials]=151; I2=37%] and 12.6% (95% CI, 9.016.2; n [4 trials]=166; I2=24%), respectively, with the use of DJBL compared with diet modification [24]. Another meta-analysis reported that patients with DJBL showed an EWL of 35.3% (95% CI, 24.646.1) at 12 months [25]. The long-term effect of DJBL is relatively underwhelming. A recent study on the long-term effect of DJBL in 15 patients followed up for a median of 42 months showed a significant reduction in absolute weight from 106.1 kg (interquartile range [IQR], 99.0128.4) at baseline to 99 kg (IQR, 88.4115.5), immediately after DJBL treatment [26]. However, the median weight increased to 102.0 kg (IQR, 94124.6) at long-term follow-up, which was not significantly different from the baseline weight (106.1 kg). Furthermore, higher frequency and severity of adverse events were reported after using the device for more than a year [27].
The DJBL has also been reported to be useful for glycemic control. A meta-analysis assessing the effect of DJBL on glycemic control in patients with obesity and type 2 DM reported a 1.3% (95% CI, 1.0ÔÇô1.6) decrease in HbA1c levels, which further decreased by 0.9% compared with the baseline value at 6 months after removing the device [28]. A recent study evaluating the cardiovascular risk in patients with type 2 DM and metabolic syndrome reported that the DJBL not only yielded weight loss and decreased the blood glucose levels but also improved the cardiovascular biomarkers, such as high-sensitive C-reactive protein (CRP), lipoprotein-associated phospholipase A2, and small dense low-density lipoprotein fraction levels (p=0.001, p<0.001, and p=0.04, respectively) [29]. The estimated overall cardiovascular risk decreased significantly after DJBL implantation, which was maintained at 69 months after device removal.
In a systematic review analyzing the adverse events of DJBL, the adverse event rate was 84.4% (mild, 75.8%; moderate, 20.5%; and severe adverse events, 3.7%) [30]. The most common adverse events are gastrointestinal disturbances, including abdominal pain and nausea, which resolve once the patient is accustomed to the presence of the device. Severe adverse events include device migration (4.9%), gastrointestinal bleeding (3.86%), sleeve obstruction (3.4%), liver abscess (0.13%), cholangitis (0.13%), acute cholecystitis (0.13%), and esophageal perforation (0.13%) [25]. DJBL has not been approved for sale by the United States FDA due to the higher incidence of liver abscess. Early device removal was reported in up to 31% of patients because of device intolerance and adverse events [31].
GASTRODUODENOJEJUNAL BYPASS SLEEVE
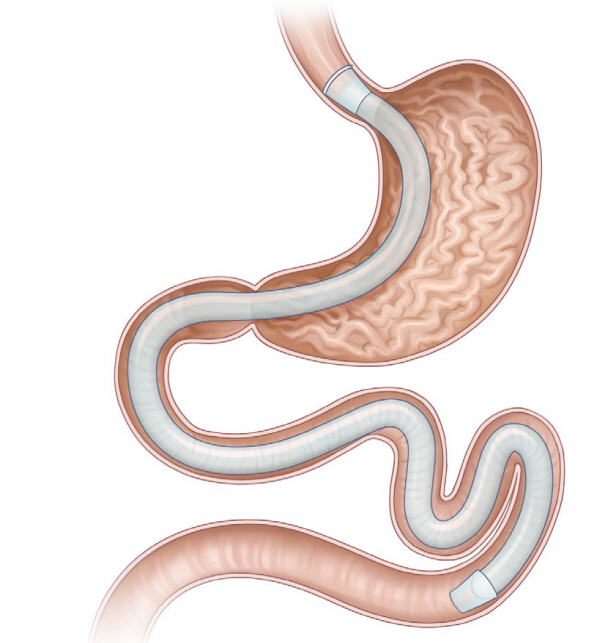
The gastroduodenojejunal bypass sleeve (GJBS, ValenTx Endoluminal Bypass; ValenTx Inc., Hopkins, MN, USA; Fig. 3) is a 120-cm-long fluoropolymer sleeve composed of a cuff, anchors, and a detachable sleeve. It is delivered endoscopically under laparoscopic guidance. It is implanted by anchoring to the gastroesophageal junction and is deployed down through the pylorus, extending the sleeve to the jejunum. The device has both restrictive and malabsorptive effects because undigested food particles pass through the lumen of the sleeve and bypass the stomach, duodenum, and proximal jejunum.
In the first trial, 24 obese patients with a mean BMI of 42 kg/m2 (range, 35.4ÔÇô50.8 kg/m2) were enrolled, and the GJBS was implanted in 22 (92%) patients [32]. Among them, 17 (77%) patients completed the 12-week treatment period and showed an average %EWL of 39.7% and an average TWL of 16.8 kg at the end of the study. Explantation was performed in 5 (23%) patients within the first three weeks due to odynophagia, and the pain resolved after explantation of the device. Of the 17 patients, 7 patients with type 2 DM who had taken hypoglycemic agents did not require medication after device implantation. In the second pilot study, the GJBS was implanted in 12 (92%) morbidly obese patients [33]. Two patients (17%) underwent device explantation due to odynphagia and dysphagia. All ten patients were followed up for 12 months, and the mean % EWL was 35.9%. Six patients who had fully attached anchors and functional devices at follow-up endoscopy showed an %EWL of 54%. Follow-up of the remaining four patients revealed a leak between the bypass device and the gastrointestinal wall, possibly a limitation of the device. Four patients with diabetes showed an improvement in fasting blood glucose values, with a mean improvement of 38%. Among the seven patients with hypertension, five showed normal blood pressure and did not require antihypertensive medication after device implantation.
DUODENAL MUCOSAL RESURFACING
Duodenal mucosal resurfacing (DMR, Revita DMR system; Fractyl Laboratories, Cambridge, MA, USA; Fig. 4) involves ablation of the superficial duodenal mucosa using a hydrothermal catheter. Recent studies showed that the duodenum is a metabolic signaling center, and the duodenal mucosa shows hyperplastic changes in response to the consumption of a high fat or sugar diet [34]. Mucosal resurfacing allows the regeneration of the normal duodenal mucosa, which is believed to correct abnormal metabolic signaling.
DMR involves submucosal expansion and circumferential hydrothermal ablation. After marking the contralateral wall of the ampulla of Vater with a clip or using argon plasma coagulation to avoid ablation of the papilla and to reduce the risk of acute pancreatitis, a guidewire is inserted beyond the ligament of Treitz. The DMR catheter is advanced over the guidewire, and circumferential mucosal lift of the post-papillary (1 cm distal to the papilla) duodenum is performed to prevent muscle layer damage. Subsequently, under endoscopic visualization, circumferential hydrothermal ablation was performed at 90┬░C for 10 s approximately 9ÔÇô10 cm from the post-papillary duodenum.
The first-in-human study of DMR included 39 patients with type 2 DM and a BMI of 24ÔÇô40 kg/m2 [35]. In this study, patients were grouped according to the length of circumferential ablation: the long duodenal segment ablation group (ÔëÑ9 cm ablation; n=28) and the short segment ablation group (<6 cm ablation; n=11). HbA1c levels were reduced by 1.2% at 6 months in the entire cohort (p<0.001). The long-segment group showed more pronounced glycemic effects at 3 months (2.5% vs. 1.2%, p<0.005) and 6 months (1.4% vs. 0.7%, p=0.30). A recent international, open-label, prospective multicenter study reported favorable 1-year outcomes of DMR and the safety of DMR use [36]. Furthermore, DMR was feasible in 80% (37/46) of the patients. The causes of DMR failure included catheter failure, difficulty in catheter tracking/positioning, duodenal tortuosity, and inadequate lifting. Modest weight loss was observed at 6 months (ÔêÆ2.5┬▒0.6 kg; p<0.001) and 12 months (ÔêÆ2.4┬▒0.7 kg; p<0.001) compared with the baseline weight. A decrease in HbA1c level of 10┬▒2 mmol/mol was observed at 6 months (p<0.001) and was maintained for up to 12 months. Moreover, fasting plasma glucose levels (ÔêÆ1.7┬▒0.5 mmol/mol; p<0.001) and insulin resistance (ÔêÆ2.9┬▒1.1; p<0.001), assessed using the homeostatic model assessment method, improved compared with the baseline values. Recently, some studies have assessed the role of DMR in liver function [37].
DMR-related adverse events were reported in 52% of the patients at 1-year follow-up, and 81% of the events were classified as mild [36]. The most common adverse events were gastrointestinal symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Malaise, fatigue, musculoskeletal pain, rash, hypoglycemia, and hyperglycemia were also reported. A procedure-related serious adverse event was reported in one patient, who developed fever (38┬░C) and malaise with an increase in CRP levels.
INCISIONLESS MAGNETIC ANASTOMOSIS SYSTEM
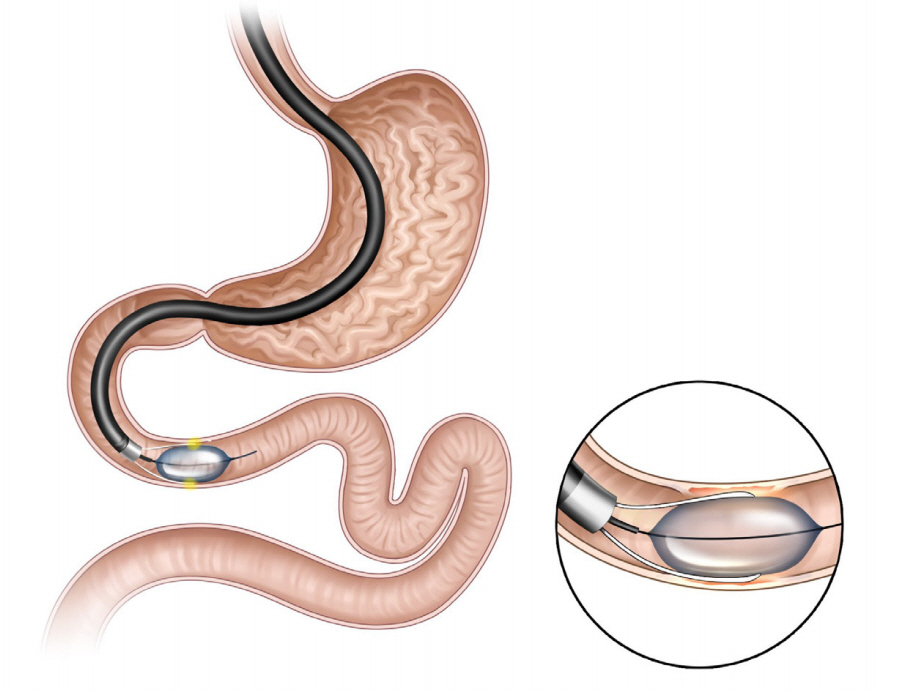
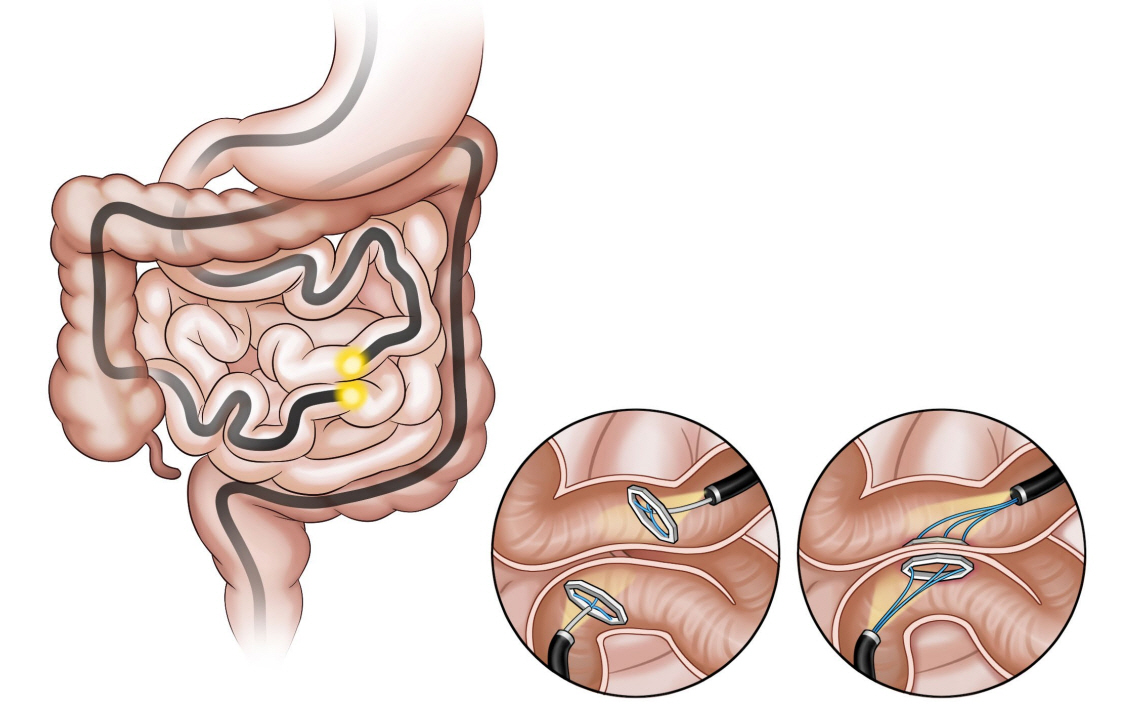
The incisionless magnetic anastomosis system (IMAS; GI Windows Inc., Bridgewater, MA, USA; Fig. 5) involves the creation of a dual-path enteral bypass using a pair of self-assembling magnets [38]. The small bowel is accessed using simultaneous colonoscopy and enteroscopy. The magnets are deployed from the working channel of each endoscope in the jejunum and ileum under fluoroscopy visualization, and the deployed magnets stick together and cause necrosis by compressing the intestinal wall. After the anastomosis is completed, the device is passed through the stool. In the initial human cases, the technical difficulties related to the use of this exclusive endoscopic approach required laparoscopic assistance.
In a single-arm human pilot study, partial jejunal diversion using IMAS was created in 10 obese patients with diabetes [39]. The IMAS was performed via colonoscopy under laparoscopic guidance, and the anastomosis remained widely patent in all patients at 1 year. The average % TWL and % EWL were 14.6% and 40.2%, respectively, at 1 year. Moreover, a significant reduction in HbA1c levels was observed in all patients with diabetes (7.8% ┬▒2.4% to 5.9% ┬▒0.58%) at 1-year follow-up. Furthermore, a significant reduction in postprandial insulin and glucose levels were observed at 2 and 6 months and a significant increase in peptide YY levels were observed at 2 months. Compared with traditional surgery, this technique allows partial flow diversion, which does not cause malabsorption, and the creation of anastomoses without suturing, thus reducing the chances of leakage.
The procedure-related adverse events reported were nausea, diarrhea, abdominal pain (including trocar site pain), and abdominal distension [39]. None of the patients experienced serious device-related adverse events.
CONCLUSIONS
There is an increase in the availability of minimally invasive treatment options for overweight, obesity, and related comorbidities. Several EBMTs that can result in moderate weight loss are available or are under investigation. Although data on long-term results are not available, they exhibited promising performance in improving glycemic control and liver function, achieving better results compared with lifestyle interventions and pharmacotherapy for weight loss, and are less invasive than surgical treatment.
Future research should use combination or sequential therapy including two EBMTs with different mechanisms of action, such as endoscopic sleeve gastroplasty and duodenal jejunal by-pass liner or an EBMT with pharmacotherapy. Moreover, these studies should focus on the appropriate selection of therapies, and a better understanding of the physiology of EBMTs is warranted to achieve better results.
As the use of EBMT is increasing worldwide, standardized training is needed to improve the clinical outcomes and safety. As obesity is a multifactorial disease, a multidisciplinary team is required to achieve satisfactory results.