INTRODUCTION
Until recently, endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) constituted the majority of advanced gastrointestinal (GI) endoscopic procedures. Although this is still the case in many centers, the last decade has seen a phenomenal increase in many other procedures, aided by advancements in technology and devices. Conditions such as morbid obesity, achalasia, and superficial cancerous and noncancerous mucosal lesions of the GI tract are regularly treated endoscopically. Some of the procedures include endoscopic submucosal dissection (ESD), endoscopic sleeve gastroplasty (ESG), and peroral endoscopic myotomy (POEM). These advanced procedures present unique challenges for anesthesia providers. While hypoxemia and pulmonary aspiration continue to test the skill and preparedness of the anesthesia provider, complications such as venous air embolus, pneumomediastinum, pneumothorax, subcutaneous emphysema, life-threatening bleeding, and perforation of the viscus pose additional problems. Anesthesia providers are expected to provide general anesthesia in an endoscopy suite and be prepared to address issues such as hypothermia and hypercarbia in an environment where little expert help is available. In short, the topic of anesthesia for advanced endoscopic procedures merits a comprehensive review.
GENERAL CONSIDERATIONS
In general, airway-related complications remain a major cause of concern. The frequency and severity of many periprocedural complications vary depending on both patient and procedural factors. ERCP remains the most common advanced procedure, and its indications have expanded. The duration of many advanced procedures is variable, and the endoscopistŌĆÖs experience plays a crucial role. In this review, we mainly focus on procedures other than ERCP and EUS.
ENDOBARIATRICS
Endobariatric procedures may be employed as a primary treatment option or in patients who regained weight after a previous bariatric surgical procedure. In some respects, the challenges are similar, except that patients presenting for revisional procedures might have lost some weight. More important, patients who previously underwent weight loss surgery may also have long-term complications, such as severe acid reflux. Clearly, anesthesia providers should be aware of all pathophysiological changes associated with obesity and the resulting anatomical changes. These changes affect pharmacology, both in terms of dosing and clinical effects.
The procedures employed as primary weight loss options are primary obesity surgery endoluminal (POSE), ESG, insertion of intragastric balloons, aspiration therapy with the AspireAssist device, and gastric botulinum toxin injection for weight loss. The revisional bariatric procedure that is typically performed for endoluminal plication in patients who had undergone a Roux-en-Y gastric bypass is called transoral outlet reduction (TORe). The gastric pouch can also be decreased in size, which is called tubular TORe or restorative obesity surgery endoluminal (ROSE). Other techniques include mucosal ablation using argon plasma coagulation of the gastrojejunal anastomosis [1].
POSE uses an incisionless operating platform to create full-thickness plications in the gastric fundus, thereby reducing gastric volume, which, in turn, limits the quantity of food that can be consumed at a given time [2]. The procedure also causes antral dysmotility that prolongs satiety by inducing an earlier and longer feeling of gastric distention. In ESG, the endoscopist attempts to remodel the greater curvature of the stomach through the placement of full-thickness sutures. Similar to POSE, this results in reduced gastric capacity and delayed gastric emptying [3,4]. Originally, ESG was performed using a superficial suction-based suturing device and had limited success owing to early suture loss. The current approach involves full-thickness suturing.
Both POSE and ESG are time-consuming procedures performed under general endotracheal anesthesia (GETA). In addition to the general risks associated with any endoscopic procedures, such as hypoxemia and aspiration, there are specific risks, including intraprocedural and postprocedural bleeding. Postprocedural pain could be substantial, requiring prolonged recovery room stay and occasional overnight hospitalization. There is an increased risk of suture release with continuous positive pressure application after the procedure. Pulmonary compliance can decrease as a result of abdominal and gastric distention due to carbon dioxide (CO2) leakage, whereas both end-tidal CO2 (EtCO2) and pulmonary resistance may increase. Although the increase in EtCO2 can be addressed by increasing minute ventilation, needle insertion into the peritoneal cavity to release excess CO2 may be occasionally required for treating pneumoperitoneum.
Insertion of an intragastric balloon is typically performed under deep propofol sedation, more commonly referred to as monitored anesthesia care (MAC). These balloons cause weight loss by inducing gastroparesis. Depending on the type of balloon employed (either gas- or fluid-filled), they can be removed under MAC or GETA. Fluid-filled balloons should be removed under GETA because of the need to puncture the balloon to release the large volume of saline to facilitate balloon removal. All balloons should be removed, as they can migrate into the small bowel and cause obstruction.
The AspireAssist device (Aspire Bariatrics Inc., King of Prussia, PA, USA) functions similar to a percutaneous endoscopic gastrostomy tube and allows the aspiration of portions of ingested food from the stomach. It has been approved by the Food and Drug Administration for use in patients with a body mass index between 35 and 55 kg/m2 [2]. Both the insertion and removal of this device are performed under deep sedation.
Gastric injection weight loss therapy involves injecting botulinum toxin into the smooth muscle of the gastric fundus to induce gastroparesis and early satiety. This is an effective weight-loss therapy in combination with diet and exercise. The procedure is performed under deep sedation.
Revisional endobariatric procedures such as TORe and ROSE utilize an endoscopic suturing device (Overstitch; Apollo Endosurgery, Austin, TX, USA) in patients who had undergone Roux-en-Y gastric bypass to narrow the gastrojejunal anastomosis and to decrease the size of the gastric pouch. These procedures are performed under GETA. Argon plasma coagulation involves the application of argon laser coagulation to the anastomotic outlet. Deep sedation is usually sufficient. Multiple sittings are needed, with each sitting taking approximately 15 min.
Postoperative nausea and vomiting (PONV) can be a troubling complication of all endobariatric procedures. Aggressive pharmacotherapy has been used to limit PONV. Additionally, postprocedural pain may be substantial, particularly after full-thickness plication procedures. However, unlike bariatric surgery procedures, endoscopic procedures do not typically require hospital admission for monitoring, pain management, or nausea control.
At the Hospital of the University of Pennsylvania, our experience is with TORe, which is performed in our outpatient facility. Anesthesia is induced with propofol and fentanyl and maintained with infusions of propofol and remifentanil (an ultrashort-acting opioid). In morbidly obese patients, the doses of both propofol and remifentanil are lower than the standard adult doses (e.g., propofol bolus of 1.5ŌĆō2 mg/kg body weight for induction). The infusions of both propofol and remifentanil are also considerably lower (approximately one-half) than those used for anesthesia maintenance in a normal-weight or overweight patient, approximately 70ŌĆō100 ╬╝g/kg/min propofol and 0.1ŌĆō0.12 ╬╝g/kg/min remifentanil. Blood pressure support often requires phenylephrine at approximately 30 ╬╝g/min. Because of the increased risk of PONV, we avoid inhalational anesthetics. Intermittent positive pressure ventilation (IPPV) is always employed, and the settings are adjusted to maintain an acceptable EtCO2. The effect of rocuronium is reversed using sugammadex. Ondansetron is routinely administered, and dexamethasone is sometimes used for PONV prophylaxis. Postprocedural pain could be substantial, and patients are maintained in the recovery room for about 1 hour before being discharged home.
POEM
POEM is performed to treat achalasia, a motility disorder of the esophagus. Gastroenterologists often misdiagnose the condition as gastroesophageal reflux disease. The absence of relaxation of the lower esophageal sphincter is a characteristic feature of achalasia. Nevertheless, this is not essential for diagnosis and is not always present [5]. The surgical treatment for this condition is a Heller myotomy, in which the anterior muscle fibers of the esophagus are incised longitudinally [6]. Laparoscopic or thoracoscopic approaches, including laparoendoscopic single-site surgery and robot-assisted myotomy, and POEM, have largely replaced open surgery [7].
The preprocedural evaluation of patients presenting for POEM should specifically focus on the increased risk of aspiration. The clinical presentation depends on the subtype of achalasia. Three subtypes are recognized: type I (classic), with minimal contractility in the esophageal body; type II, with intermittent periods of panesophageal pressurization; and type III (spastic), with premature or spastic distal esophageal contractions. Progressively worsening dysphagia initially to solids, followed by both solids and liquids, is the leading presentation [8]. Severe reflux, history of regurgitation, pneumonia, and weight loss are signs of possible preprocedural/intraprocedural aspiration. Aspiration pneumonitis (acute and chronic), diffuse aspiration bronchiolitis, isolated bronchospasm, and aspiration pneumonia can occur in these patients. Chronic interstitial fibrosis is conceivable over a period of time and is related to periodic aspiration [9].
In terms of anesthetic management, GETA is required for POEM [10]. Extended fasting time is often required (up to 48 h, depending on the degree of symptomatology, previous esophagogastroduodenoscopy [EGD] findings, and manometric studies) to minimize the risk of aspiration. Rapid sequence induction and intubation, often in a slight head-up position, has the least risk of aspiration. Even though suuden airway obstruction is rare, it can be caused by tracheomalacia, related to posterior tracheal cartilage ischemic damage resulting from chronic pressure due to massive esophageal dilation. The collapse is dynamic in nature, which can be relieved by IPPV with positive end-expiratory pressure. Cuffed reinforced endotracheal tubes are preferred to avoid kinking or obstruction of the endotracheal tube during endoscopy. In addition to standard monitoring, neuromuscular monitoring to ensure an appropriate degree of paralysis is essential. An increase in EtCO2 is anticipated and attributed to the use of CO2 by the endoscopist and its absorption. Any unexpected movement will not be appreciated by the endoscopist and should be avoided. The availability of sugammadex allows an anesthesia provider to err on the side of caution rather than risk inadequate paralysis. The procedure itself involves making a mucosal incision in the mid-esophagus, entering it, and creating a submucosal tunnel all the way to the gastric cardia by using a forward-viewing endoscope with a transparent distal cap and an ESD knife. Once the muscle fibers are exposed, they are incised and the mucosal incision is closed with endoscopic clips [11].
Intraprocedural complications include pneumothorax, mediastinal emphysema, subcutaneous emphysema, pneumoperitoneum, and, rarely, capnopericardium. To diagnose these complications, a high degree of suspicion is necessary [12]. The postprocedural period may be complicated by delayed hemorrhage, pleural effusion, minor inflammation or segmental atelectasis of the lungs, and gas under the diaphragm or aeroperitoneum.
Nearly 25% of patients undergoing POEM experience asymptomatic pneumothorax, especially when the procedure is performed under GETA. Positive pressure ventilation increases the air escape. Dissection of the thoracic portion of the esophagus and the resulting mediastinal pleural tear are the likely causes. Clearly, the use of air for insufflation must be avoided, which, despite potentially providing better distension and more manipulative space, is also associated with higher morbidity and mortality than the use of CO2.
Although a rare complication, capnopericardium can cause cardiac arrest. Disappearing EtCO2 tracing or pulse tracing on a plethysmogram may be the first indicators of capnopericardium. Blood pressure may be nonrecordable, and electrocardiogram changes might appear, including life-threatening arrhythmias. Immediate suspension of the procedure and endoscope withdrawal along with cardiopulmonary resuscitation may be required. Transthoracic echocardiography may not adequately image the heart. The procedure may need to be aborted, as any suturing of the mucosal incision might worsen the capnopericardium.
Subcutaneous emphysema is another known complication. There may be a palpable crepitus. CO2 insufflation may become challenging with both flow and pressure. An increase in EtCO2 may be noticed along with respiratory acidosis during blood gas measurement. Lung compliance will decrease (with increased airway pressure) along with sinus tachycardia and other cardiac arrhythmias. An increase in blood pressure is the likely result of an increase in partial CO2 >50 mm Hg [13]. Decompression by inserting a 14- or 16-gauge angiocatheter in the right lower abdomen, approximately 5 cm below the costal margin, preferably guided by ultrasound, may become necessary. The presence of air in the mediastinum may not pose considerable clinical challenges and is typically observed along with subcutaneous emphysema. An increase in EtCO2, a decrease in saturation of peripheral oxygen (SpO2), and inability to archive the desired tidal volume despite maximal safely attainable manual ventilation are key indicators of pneumomediastinum. The endoscope should be withdrawn, and the patient should be evaluated. Malignant hyperthermia should be excluded, although difficulty in ventilation and lack of hyperthermia can eliminate such a possibility. Acidosis will be seen on arterial blood gas, despite the administration of 100% oxygen. Hypotension, cardiac arrhythmia, and hemodynamic collapse are possible. Chest radiography might demonstrate variable pneumomediastinum and subcutaneous emphysema. De-aeration might become necessary and can be accomplished with a percutaneous abdominal needle along with positive pressure ventilation with suitable ventilator settings [14].
All of the above complications may appear for the first time in the postprocedural period. At our hospital, patients presenting for POEM fast longer than usual and stay on clear fluids for 48 hours. We used to perform this procedure in our inpatient facility; however, after gaining more experience, we now perform about half of such procedures in our outpatient facility and the patients are usually discharged the next day. General anesthesia with propofol and fentanyl (generally 2ŌĆō3 mg/kg and 100ŌĆō150 ┬Ąg, respectively), followed by intubation facilitated with either succinylcholine or rocuronium (usually 100 mg for both) as a rapid sequence induction intubation technique, is used. Anesthesia is maintained with propofolŌĆōremifentanil (infusion at 120ŌĆō150 ┬Ąg/kg/min and 0.2ŌĆō0.25 ┬Ąg/kg/min, titrated). CO2 accumulation is inevitable, and higher tidal volumes during IPPV are often needed. The incidence of PONV is higher than that with routine EGD, and prophylaxis with ondansetron is a standard practice. The procedure usually takes 1ŌĆō2 hours.
ESD
ESD can be performed for en bloc resection of early GI luminal cancers. This procedure is performed on localized lesions with a minimal risk of metastasis [15,16]. Unlike endoscopic mucosal resection, which can only remove lesions in a piecemeal fashion, ESD is intended to treat larger lesions with en bloc resection to assess the tumor-free margins. As a result, this procedure has a higher rate of complications, such as perforation causing peritonitis and delayed bleeding.
The endoscopist expects a relatively motionless and well-sedated patient. At our center, for lesions in the lower GI tract, ESD is generally performed under deep sedation with propofol. Occasionally, the procedure can take 2ŌĆō3 hours. A short-acting opioid, such as fentanyl, is often required in increments and may contribute to longer recovery and postprocedural stays.
However, for upper GI lesions, GETA is preferred because of the risk of intraprocedural bleeding. It also ensures a stable working field without rapid or unexpected movements. It has been reported that the rate of curative resection is better and the perforation rate is lower in ESD performed under GETA than in ESD performed under conscious sedation after adjusting for several clinicopathologic factors, including the experience of the endoscopist, thereby improving oncologic outcomes in patients with superficial esophageal squamous cell carcinoma [16].
At our hospital, general anesthesia with an endotracheal tube is always employed for ESD procedures. The induction doses of propofol and fentanyl are similar to those used in POEM; however, rapid sequence induction is not generally required. The maintenance of anesthesia is also similar. The procedure takes approximately 1ŌĆō2 hours, and occasionally longer, depending on the size of the lesion. CO2 accumulation requires a higher tidal volume with IPPV. PONV prophylaxis with ondansetron is a standard practice. Postprocedural pain management may require an overnight stay.
ERCP
ERCP is a routine advanced procedure performed in many academic and freestanding endoscopy units. Although the issue of airway management is still unsettled, the choice is largely left to the institution and the individual anesthesia provider. Other reviews on this topic have extensively discussed the related issues [17,18].
Cases of fatal and nonfatal air embolisms during ERCP have been reported [19-24]. Other procedures such as EGD, EUS, colonoscopy, or sigmoidoscopy have also been associated with air embolism [25,26]. A high index of clinical suspicion is required for diagnosis, and aggressive treatment is needed to avoid a fatal outcome. The use of precordial Doppler ultrasound and transesophageal echocardiography is helpful for establishing a diagnosis of air embolism. Besides traditional supportive measures, hyperbaric oxygen therapy should be considered in cases of suspected cerebral air embolism to improve neurological outcomes. Air embolism can also manifest for the first time after the procedure. A case of ventricular fibrillation requiring prolonged cardiopulmonary resuscitation and resulting in death in the recovery room has been reported [19]. As previously stated, it is crucial that CO2 is used for advanced endoscopic procedures such as ERCP to decrease the risk of air embolism.
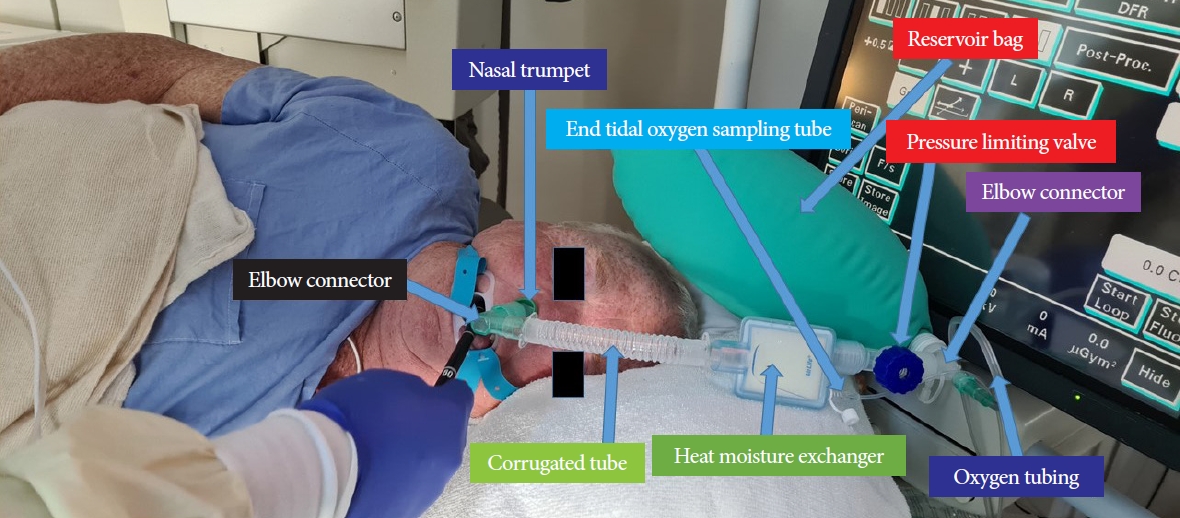
At our hospital, >90% of ERCP procedures (including nearly 100% of procedures in the outpatient center) are performed under anesthesiologist (who is always a physician)-supervised deep propofol sedation administered by a certified nurse anesthetist. Propofol and fentanyl are typically administered as 80ŌĆō100 mg and 25ŌĆō50 ┬Ąg boluses, respectively, followed by the infusion of 80ŌĆō120 ┬Ąg/kg/min propofol. The patients breathe spontaneously, and supplemental oxygen is generally administered through a nasal trumpet introduced into the nose or mouth (Fig. 1).
EUS-GUIDED DRAINAGE OF PERIPANCREATIC COLLECTIONS
EUS-guided drainage of large pancreatic pseudocysts and pancreatic walled-off necrosis to allow for transgastric necrosectomy are rare procedures. These procedures are performed in patients with pancreatitis and typically in an inpatient facility, although they can also be performed in an outpatient facility.
Ultrasound-guided drainage of pancreatic cysts, including pseudocysts, may be performed under deep sedation if the cysts are small and have a negligible risk of pulmonary aspiration resulting from the leak/flooding of the stomach. However, most pseudocysts that require drainage are large and contain a large volume of fluid that will be released into the GI lumen. As there is a risk of pulmonary aspiration with large cysts, GETA tube is advisable.
Patients with infected peripancreatic walled-off necrosis collections will also require drainage and multiple sessions of direct endoscopic necrosectomy. Patients with severe necrotizing pancreatitis can develop a well-demarcated, organized collection of necrotic tissue [27]. A transgastric stent can be placed into the collection to facilitate direct endoscopic necrosectomy for the endoscopic debridement of this collection. This approach is known to result in a lower incidence of new-onset multiorgan failure and a lower major complication rate than surgical necrosectomy. In contrast to open surgical necrosectomy, direct endoscopic necrosectomy is associated with lower rates of complications such as bleeding and inadvertent puncture of the adjacent viscera, as well as with a decreased risk of chronic pancreaticocutaneous fistulae. This procedure is always performed under GETA.
We recognize that the anesthesia/sedation practice for advanced procedures, especially ERCP, varies worldwide. In most countries (outside the United States), the majority of ERCP and EUS procedures are performed either with intravenous conscious sedation or a registered nurse (not a nurse anesthetist)-administered propofol sedation. Generally, the propofol doses are lower. As a likely result, the satisfaction of both endoscopists and patients is lower. Nonetheless, the practice is safe and, in fact, a meta-analysis has shown it to be safer than physician anesthesiologist-administered propofol sedation [28]. For the advanced procedures discussed here, with the exception of uncomplicated ERCP and EUS, we recommend that the sedation be administered or supervised by an anesthesiologist.
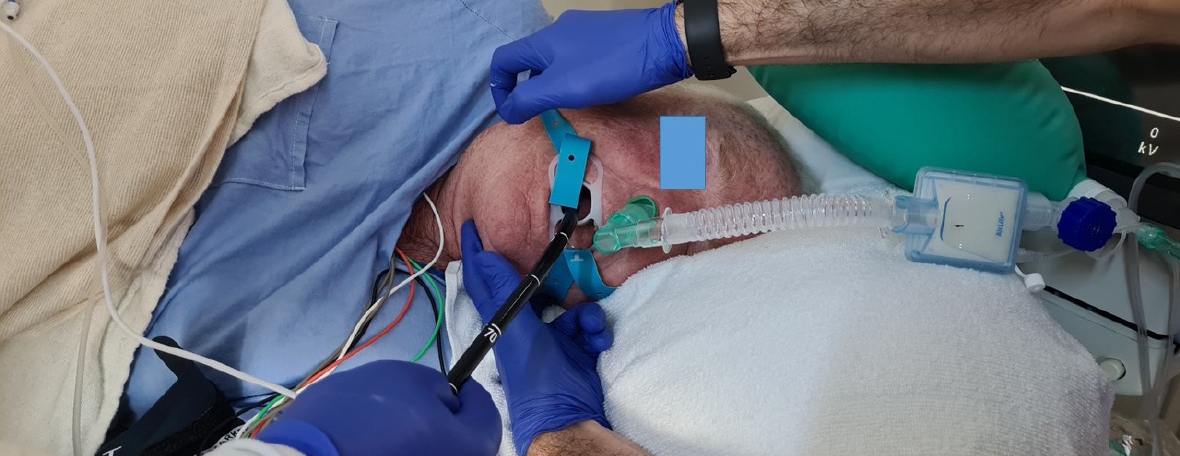
Irrespective of the person administering the sedation, adverse events such as desaturation are inevitable. The most common causes of desaturation are hypoventilation (as a result of sedative medications) and upper airway obstruction. An attentive clinician/nurse will be able to recognize the cause. The corrective measures depend on the cause. Airway obstruction often responds to measures such as chin lift, jaw thrust, and neck extension ŌĆō maneuvers often not employed or employed late (Fig. 2). The oxygen flow should be immediately increased to 10ŌĆō15 L/min. If these measures are ineffective, the endoscopist must withdraw the endoscope, and bag mask ventilation should begin in earnest. If laryngospasm is recognized, the short-acting muscle relaxant succinylcholine should be administered at approximately 25ŌĆō50 mg, early than late. Certain factors increase the risk of hypoventilation and airway obstruction. Elderly patients are particularly susceptible to hypoventilation, even with small doses of sedative medications. Smokers, obese individuals, those with obstructive sleep apnea, patients with chronic obstructive pulmonary disease, and those recovering from a recent upper respiratory infection are susceptible to upper airway obstruction, whereas patients with asthma are predisposed to bronchial obstruction.
CONCLUSIONS
In conclusion, advanced GI endoscopic procedures continue to evolve and pose many unique challenges to the anesthesia provider. Endoscopists are also likely to be in a ŌĆ£learning modeŌĆØ during the early stages of the introduction of these procedures in their procedure settings. A discussion between the anesthesia provider and the endoscopist is crucial to recognize the risks and preempt them. As always, the team should be prepared to manage both expected and unexpected adverse events.