INTRODUCTION
The role of endoscopy is central to the evaluation and management of inflammatory bowel disease (IBD), especially in the diagnosis, tissue acquisition, treatment-response evaluation, surveillance of colorectal dysplasia, and monitoring of mucosal healing (MH).1-3 In addition, endoscopy is also used for therapeutic purposes.4 Although achieving clinical remission is considered the goal of IBD treatment, endoscopic disease activity might last even in the clinical remission state.5-9 Hence, there remains a risk of under-or over-treatment when management strategies are determined solely based on symptoms. As endoscopic remission or MH is significantly associated with favorable clinical outcomes, more emphasis has been placed on achieving it, as a treatment target for IBD.10,11 Recent advancements in biologics have made MH realizable, resulting in a paradigm shift in IBD management goals away from clinical remission and toward MH.12 MH, being relatively more objective and quantifiable than clinical remission, is a superior treatment endpoint.
However, for accurate assessment of MH, standardized systems are needed to report the different gastrointestinal mucosal appearances in IBD. Various endoscopic activity indices have been developed to enable physicians to interpret endoscopic findings and translate them into validated scores that grade disease activity. Such indices have been introduced for ulcerative colitis (UC) and Crohn's disease (CD). Each index has its individual strengths and weaknesses; however, most remain to be fully validated due to differences in the parameters employed and the inter-observer agreements.1 Herein, we aimed to review the features, strengths, and weaknesses of the most common endoscopic activity indices for IBD, their clinical significance, and the factors to be considered for their practical application.
ENDOSCOPIC DISEASE ACTIVITY INDICES IN ULCERATIVE COLITIS
Ever since Truelove and Witts introduced the first UC severity scoring system in 1955, various other endoscopic scoring systems for UC have been developed.13 Although each scoring system differs slightly, they all score the mucosal activity in UC in terms of erythema, edema, loss of fine vascular pattern, and granularity.14 In addition, mucosal friability, spontaneous bleeding, and ulceration have also been evaluated and are considered to indicate severe disease.14 Truelove and Witts classified the gastrointestinal lesions as normal or near-normal, improved, or no change/worse based on these endoscopic findings.13 Among the various scoring systems, this review focuses on ones that are currently the most commonly used in trials or practice: the Mayo endoscopic sub-score (MES) and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS), which is a more recently validated system.
The MES was first introduced by Schroeder et al.15 as a component of the Mayo Clinical Activity Index. The MES provides a score ranging from 0 to 3 based on four mucosal descriptors, including erythema, friability, vascular pattern, and erosions or ulceration by definition (Table 1). Although it has not yet been validated, MES has been widely used in clinical trials and routine practice owing to its ease of use.16 However, it only describes the most severely involved segment and does not reflect disease extension or focal healing. Moreover, in MES, friability has been listed as a component of both mild and moderate disease activity, leading to the overlapping of patients between the two levels of disease severity and is thus a limitation of the scoring system. Inter-observer variability is another limitation of MES.17 For overcoming these limitations, a modified Mayo Clinic Endoscopic Score has been developed and applied in several studies.18-20 In this updated system, the total sum of the scores of the five segments of the colon is calculated and then multiplied by the number of intestinal segments involved.21 Yet, this system remains to be validated.1
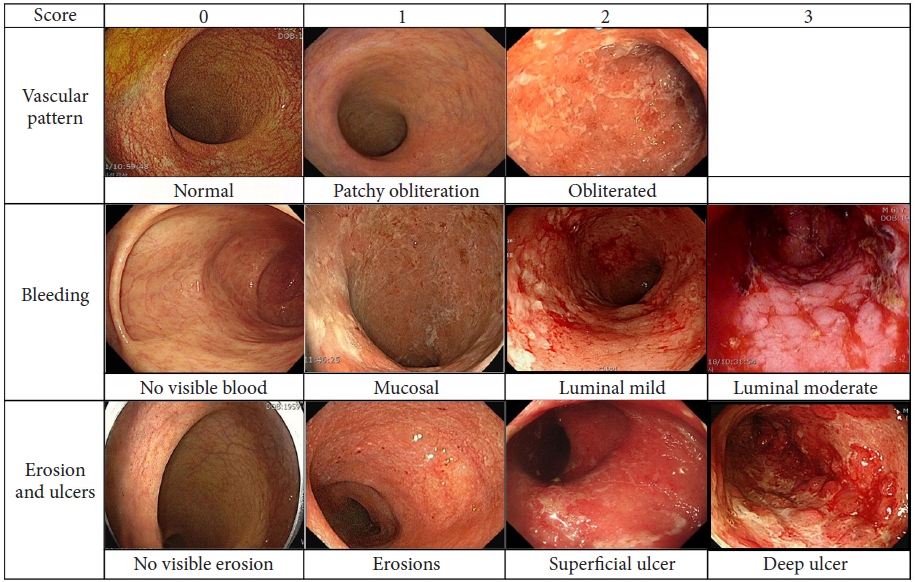
In 2012, another scoring system was prospectively developed by Travis et al.,22,23 known as the UCEIS, which employed the sum of the scores of three mucosal descriptors, including vascular pattern (scored 0-2), bleeding (score 0 to 3), and erosion/ulcers (score 0 to 3) (Fig. 1). Each score reflects the most severe lesion; hence, microscopic involvement cannot be determined.1 This system can be easily applied in routine clinical trials and practice, although a moderate inter-observer agreement has been reported.23 In comparison to MES, the UCEIS scoring system is more objective, has more specificity, and has better sensitivity for UC disease activity.24 Although each study uses its own definitions, there is no validated definition of endoscopic remission or criteria for categorizing mild, moderate, and severe UC in the UCEIS system.16
ENDOSCOPIC ACTIVITY INDEX FOR CROHN'S DISEASE
It is more challenging to develop an endoscopic index for CD that can accurately assess mucosal lesions in CD because, unlike UC, CD involves multiple locations and phenotypes.25,26 To date, two validated endoscopic scoring systems exist for CD. The first system is the Crohn's Disease Endoscopic Index of Severity (CDEIS), reported in 1989 by the Groupe dŌĆÖEtudes Therapeutiques des affections Inflammatoires du tube Digestif27 which was validated prospectively and reproducible with a good inter-observer agreement.24,28,29 The CDEIS includes parameters such as the presence of superficial or deep ulcers and the percentage of ulcerated or affected surfaces. These parameters were individually scored for the five colon segments (ileum, right colon, transverse colon, left and sigmoid colon, and rectum), summed, and then divided by the number of segments involved. An additional score was provided for cases with stenosis (ulcerated or non-ulcerated in each segment).27 The total score ranged from 0 to 44 and has been accepted as the gold standard for assessing endoscopic disease activity in CD with high sensitivity. CDEIS correlates well with disease activity.27 Although not formally validated, CDEIS scores Ōēż7 are considered to denote endoscopic resolution, while CDEIS scores Ōēż3 as complete resolution.1 Nevertheless, CDEIS is considered weak because it is too complex to assess and calculate, and training is needed to evaluate the extent, affected surface, and depth of the ulcer.
To overcome the complexity of CDEIS, the Simple Endoscopic Score for CD (SES-CD) was developed in 2004.30 The SES-CD evaluates ulcers based on size and affected ulcerated surfaces. The affected intestinal surfaces and the presence of stenosis were scored from 0 to 3 in all five segments and summed.30 These two endoscopic scores in CD correlated well with each other.30
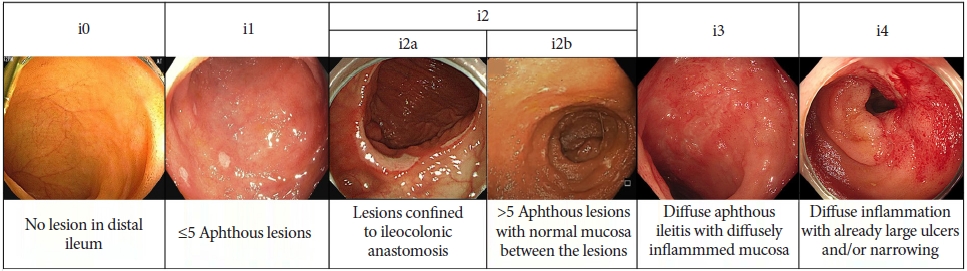
The Rutgeerts et al.31 postoperative endoscopic index is the only endoscopic activity index available for postoperative CD and was developed in 1984.32 The scores were used to evaluate endoscopic recurrence in the neoterminal ileum after ileocolic resection based on the extent of the aphthous ulcer, large ulcer, or luminal narrowing in the ileum from i0 to i4 (Fig. 2). Although not fully validated, a Rutgeerts score of 3 or 4 within one year of ileocolic resection indicated a higher risk of clinical recurrence after three years than in those with 0 or 1 (> 90.1% vs. 10% to 15%).32 Meanwhile, i2, defined as more than five aphthous ulcers in the neoterminal ileum or lesions confined to the ileocolonic anastomosis, showed conflicting clinical outcomes, and the risk of recurrence in anastomotic lesions was uncertain.33 Therefore, a modified Rutgeerts score, which subdivides i2 lesions into i2a (isolated anastomosis lesion) and i2b (>5 aphthous ulcers in the neoterminal ileum), has been suggested (Fig. 2).34 A recent retrospective study showed that i2a did not increase the risk of disease progression compared with i2b.35 Table 1 summarizes the included endoscopic descriptors, inter-observer agreement, strength, and weakness of common endoscopic indices used to evaluate UC and CD severity.
WHY DOES ENDOSCOPIC ACTIVITY MATTER IN INFLAMMATORY BOWEL DISEASE?
Although clinical symptoms do not always correlate with endoscopic activity, the clinical outcomes of IBD can be predicted based on endoscopic severity. In patients with acute, severe UC, the incidence of colectomy was significantly higher in patients with UCEIS scores >5 than in those with lower UCEIS scores (p=0.012).36 The presence of severe lesions as determined by endoscopy increased the risk of colectomy in patients with CD from 6% to 31% after 12 months of examination.37
Recently, many studies have demonstrated that MH is associated with prolonged remission, reduced risk of hospitalization, and surgical procedures.38-44 MH reduces features of bowel damage in CD, such as fistula development, the requirement of immunosuppressive agents, and the risk for colorectal cancer.45-48 In particular, patients with UC in endoscopic remission had a similar risk of colorectal cancer as that of the general population.49 In addition, MH can improve the quality of life.11 In this context, a patient with Rutgeerts score i0 or i1 showed a significantly lower risk of clinical recurrence postoperatively.32 Based on this evidence, the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) has recommended closely monitoring the disease activity to achieve endoscopic remission as a long-term treatment target.12
HOW TO APPLY ENDOSCOPIC ACTVITY IN PRACTICE
When to assess?
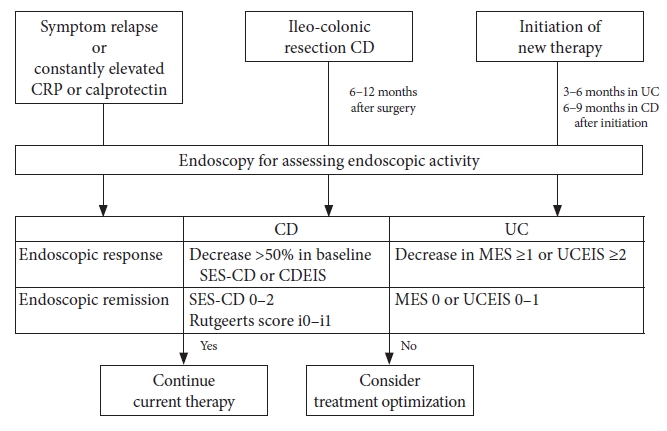
The timing of endoscopy is essential for predicting long-term prognosis through mucosal evaluation. Endoscopic assessment should be considered when aggravated clinical symptoms and elevated inflammatory markers, such as C-reactive protein or calprotectin, are constantly observed as well as at the time of initial diagnosis.16 In these cases, endoscopy provides critical information for appropriate clinical decision-making. With a particular emphasis on achieving MH, follow-up endoscopy at 3 to 6 months after starting the therapy is suggested for assessing the treatment response.16 As ileocolonoscopy within 6 to 12 months after ileocolectomy is known to be a significant predictor of long-term outcomes; follow-up endoscopy at this time is recommended in CD patients who underwent ileocolonic resection.32
Which index is better to use?
Although the assessment of endoscopic activity is essential for predicting clinical outcomes, its routine application in practice is limited for several reasons. First, in the case of CD, both CDEIS and SES-CD are complex and time-consuming. Second, a consensus regarding the best index for assessing endoscopic activity in both CD and UC is currently unavailable. There are many studies regarding endoscopic activity and clinical outcomes; however, the index used may vary, and there is no clarity regarding which system is more accurate. Recently, one study compared the MES and UCEIS in patients with acute severe UC in whom tacrolimus was used. Both UCEIS and MES significantly decreased after tacrolimus induction. However, when they considered the score change in remission, responder and non-responder only the UCEIS score decreased in responders, whereas there was no significant change in the MES. Based on these results, the UCEIS was concluded to reflect the clinical outcomes more accurately than the MES.50 There are no comparative studies between these two indices with respect to CD; however, considering that the SES-CD requires fewer calculations, it could be easier to use than CDEIS. Therefore, the updated Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) II guidelines recommend using SES-CD for CD patients and MES or UCEIS for assessing disease activity using endoscopy.12
How much healing do we need to improve clinical outcomes?
A validated standard definition of endoscopic response or remission has not yet been reported. Recently, members of the IOIBD have developed a consensus definition in clinical trials using the Delphi process among IBD experts. In UC, a decrease in MES Ōēź1 or UCEIS Ōēź2 was suggested as an endoscopic response, and a UCEIS score of 0 was considered to indicate endoscopic remission.51 However, these definitions were used in clinical trials and could differ from those appropriate in routine practice. Therefore, more studies are needed to define predictive values for improved long-term outcomes. In the case of CD, a decrease in the CDEIS or SES-CD by >50% was considered an endoscopic response,52 based on the results of a post-hoc analysis of the Study of Biologic and Immunomodulator Naive Patients in Crohn's Disease trials, which reported that a >50% decrease in both SES-CD and CDEIS scores at 26 weeks was a significant predictor of corticosteroid-free clinical remission at 50 weeks.53 They also suggested that achieving an SES-CD score of 0-2 indicates endoscopic remission. In postoperative CD, a Rutgeerts score of i0-i1 was suggested to predict endoscopic remission after ileocolectomy.52
Although many studies support that MH can improve clinical outcomes, the degree of healing required for predicting better outcomes remains controversial. According to the ACT 1 trial, the corticosteroid-free remission rates at weeks 30 and 54 were significantly different from those with the cut-off MES score of 1 at week eight after treatment with infliximab. In addition, colectomy-free survival was substantially higher in patients with MES of 0 to 1 than in those with an MES score >1.5 Another meta-analysis also reported that the risk of colectomy was not significantly different between complete and partial MH in UC and CD.54 Meanwhile, Barreiro-de Acosta et al.55 recently evaluated whether the risk of relapse increases as the MES increases from 0 to 1. The results showed that relapse within five months occurred more frequently in patients with MES of 1 (36.6%) than in those with MES of 0 (9.4%, p<0.001), and MES of 1 was the only independent factor associated with UC relapse (odds ratio, 6.27; 95% confidence interval, 2.73ŌĆō14.4; p<0.001).
Although more evidence is needed to confirm, experts in STRIDE II reached a consensus regarding the target definition of endoscopic healing as a score in SES-CD <3 or absence of ulceration in CD and MES=0 or UCEIS Ōēż1 in UC.12 Based on studies that show the benefits of optimizing treatment to target MH,54,56,57 they also recommend escalating treatment if the previous treatment could not achieve the target.12 The application of endoscopic assessment in clinical practice is summarized in Figure 3.
CONCLUSIONS
Evaluation of endoscopic activity has become crucial in IBD management because MH has been associated with favorable long-term clinical outcomes. Although numerous endoscopic scoring systems are currently available, for clinical research and routine practice, the MES and UCEIS are used in UC, the CDEIS and SES-CD are employed in CD, and the Rutgeerts score is used in post-ileocolonic resection. The application of these scoring systems in clinical settings is limited because of their complexity, inter-observer variability, lack of validation, and lack of consensus regarding the definition of disease severity. Moreover, the degree of MH necessary to improve long-term outcomes remains unclear. However, endoscopic assessment has become an important standard of care, and we need to fully understand each endoscopic activity index and apply it in practice after accurate interpretation. We also need to optimize the treatment to achieve MH for better patient outcomes.