INTRODUCTION
Enteritis cystica profunda (ECP) is a lesion in which a mucin-filled cystic space is surrounded partially with non-neoplastic epithelial cells in the submucosal tissue of the small bowel.1 The cause of ECP is not certain but congenital factors as well as acquired factors such as intestinal inflammation are suggested as the causes.2 ECP is a small intestine lesion sharing similar histological characteristics with colitis cystica profunda (CCP), but is reported more rarely than CCP.1,3 According to previously reported cases, it was accompanied commonly by underlying diseases such as Crohn's disease and Peutz-Jeghers syndrome, and most of the cases occurred in the ileum and the jejunum.3 In Korea, three cases have been reported so far, including one case accompanied by perforation of the jejunum in a Peutz-Jeghers syndrome patient,4 one case that occurred in the duodenum and was accompanied by gastric adenocarcinoma,2 and one case that occurred in the duodenum without underlying diseases.5 We experienced a case of ECP which was suspected to be choledochocele at first because the lesion developed in the form of cystic mass on the ampulla of Vater without any particular underlying disease, but in histopathologic examination after endoscopic removal of the lesion it was diagnosed as ECP. So we report this here along with the literature review because it was considered a very rare case.
CASE REPORT
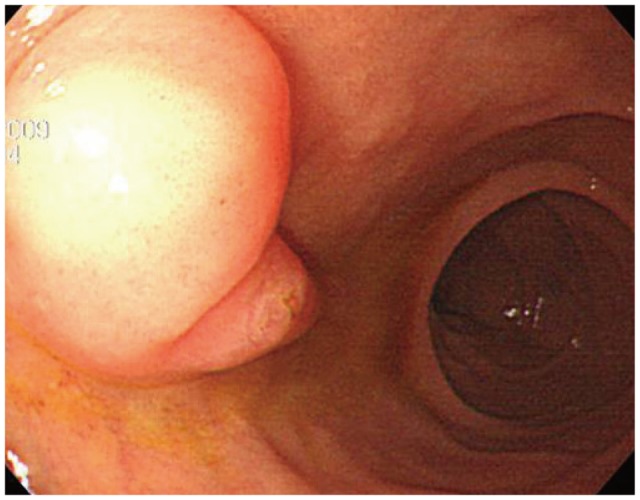
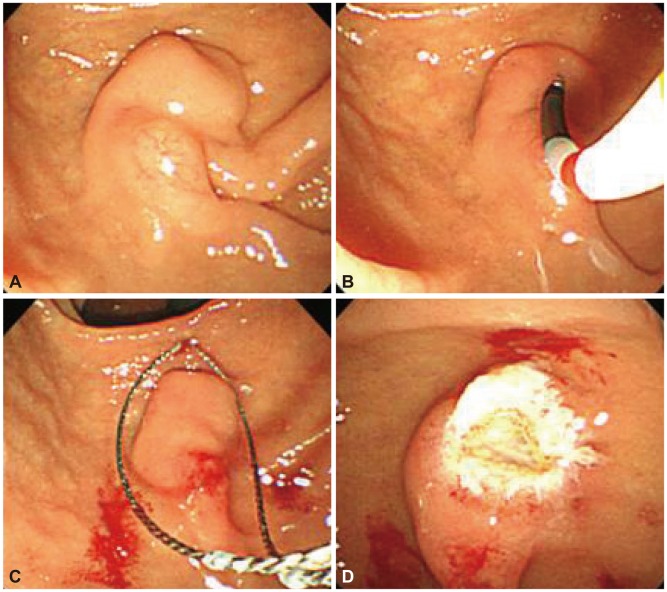
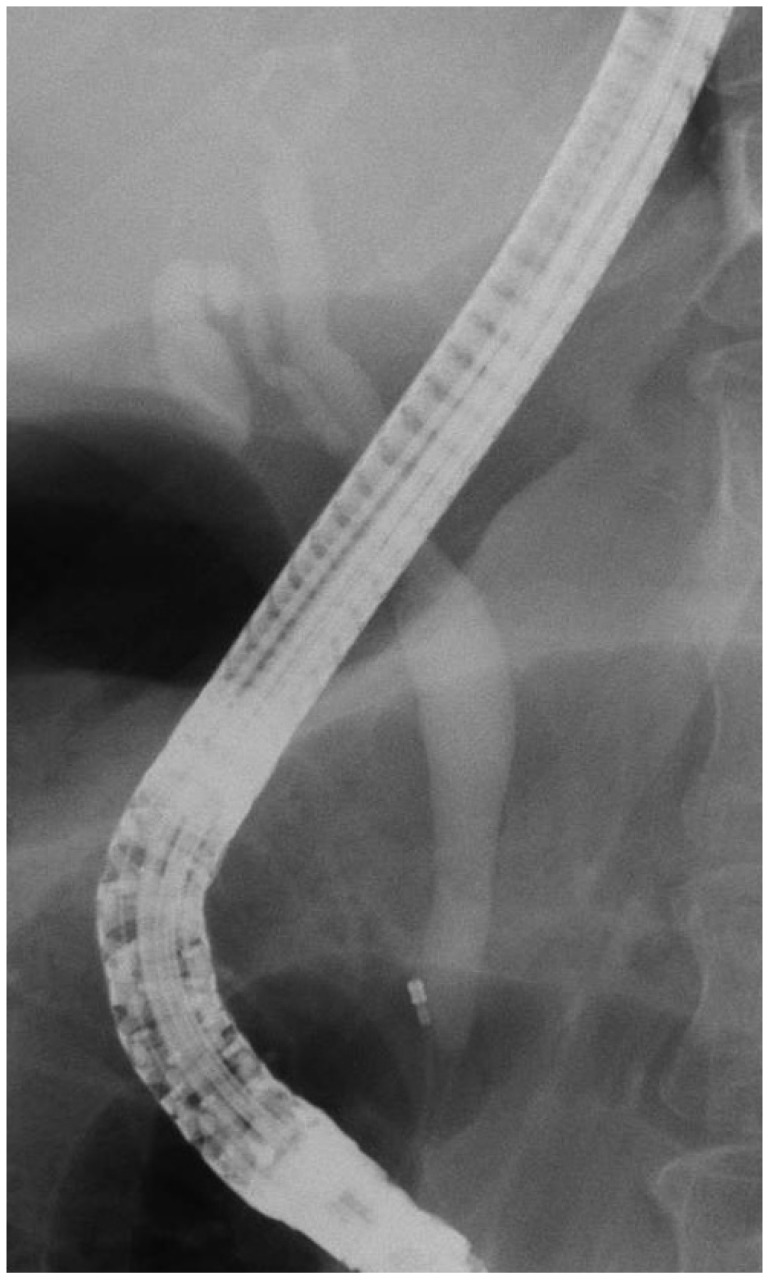
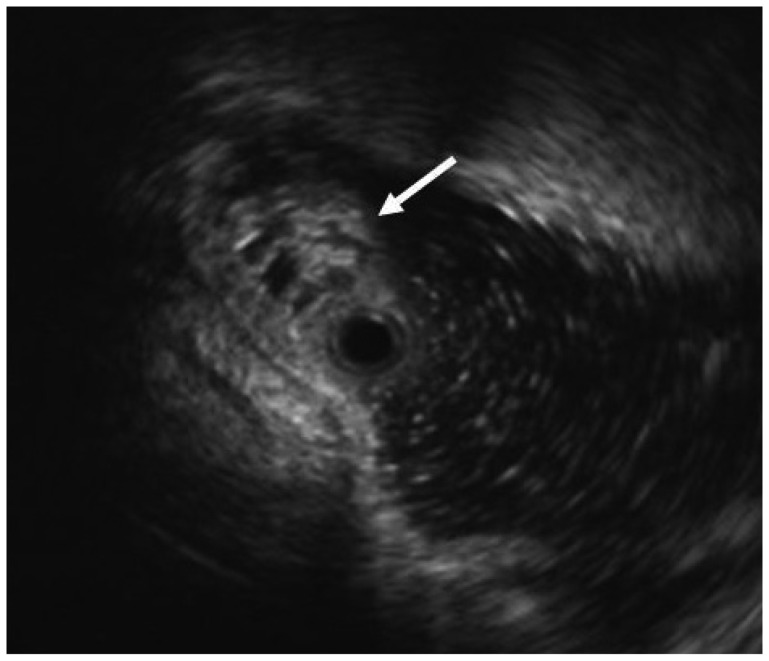
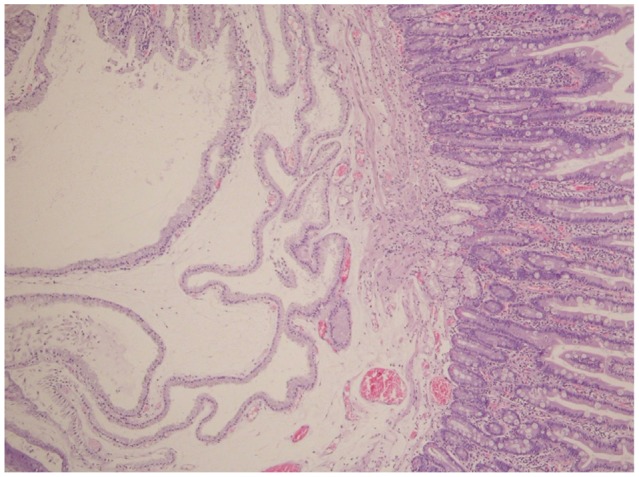
The patient, a 58-year-old woman, visited the hospital for health examination without any particular symptom, and esophagogastroduodenoscopy (EGD) showed about 1 cm-sized smooth protruding lesion on the ampulla of Vater of the duodenum (Fig. 1). She had received surgical resection for uterine myoma 10 years before. There was no noteworthy fact in her family history and social history. On her visit, vital signs were measured as blood pressure 120/80 mm Hg, pulse rate 70/min, respiration rate 18/min, and body temperature 36.5ā. She had clear consciousness and healthy appearance. At physical examination, anemic conjunctiva, icteric sclera, cervical lymph nodes were not noted. Chest auscultation did not show any abnormality, and heart sound was normal. There was no tenderness, palpable mass and bowel sound was normal. There was no particular finding in limb and neurological examination. In peripheral blood test, white blood cell was 6,900/mm3, hemoglobin 13.9 g/dL, and platelet count 233,000/mm3. The results of blood chemistry were aspartate aminotransferase/alanine aminotransferase 21/27 IU/L, alkaline phosphatase 61 IU/L, total protein 6.7 g/dL, albumin 4.3 g/dL, total bilirubin 0.7 mg/dL, amylase 39 U/L, lipase 241 U/L, blood urea nitrogen 16.0 mg/dL, and creatinine 0.8 mg/dL. The results of immunoassay test were hepatitis B surface antigen negative, hepatitis B surface antibody positive, and hepatitis C virus antibody negative, and serum tumor markers were alpha fetoprotein 1.7 ng/mL, carcinoembryonic antigen 0.9 ng/mL, and carbohydrate antigen 19-9 2.30 U/mL. In duodenosocpy, about 1 cm-sized softly protruding submucosal tumor covered with normal mucosa was observed in the proximal part of the major papilla of the ampulla of Vater. The tumor sank softly and then elevated again when pressed with biopsy forceps, and the major papilla was normal (Fig. 2A, B). As choledochocele was suspected, we performed magnetic resonance cholangiopancreatography (MRCP) but the dilation of the common bile duct surrounding the papilla or a cystic lesion connected to the common bile duct was not observed. In the results of endoscopic retrograde cholangiopancreatography (ERCP), when contrast agent was injected, the dilation of the papillary part or a cystic lesion connected to the common bile duct was not observed, and anomalous pancreaticobiliary ductal union or dilation was not observed either (Fig. 3). Thus, with excluding the possibility of choledochocele, we suspected submucosal tumor and performed endoscopic ultrasonography (EUS). In the results, about 8 mm sized anechoic lesion having multiple septa was observed in the submucosal layer (Fig. 4). For pathologic confirmation, the subepithelial lesion was resected by snare polypectomy with papillary orifice being preserved under the side viewing endoscopy (Fig. 2C, D). In histopathologic examination, a number of cysts lined with mucous columnar epithelia were observed in the submucosal layer, and because dimorphism of cystic epithelial cells was not observed, the case was diagnosed as ECP (Fig. 5). As the patient was healthy and there was no other abnormal finding on abdominal magnetic resonance imaging and EGD, the patient was discharged without any further evaluation and has been followed up without any notable symptom for 1 year.
DISCUSSION
ECP is a very rare disease occurring in the small intestine and is characterized by a mucin-filled cystic lesion in the mucosal layer, submucosal layer, and muscularis propria.6 Lesions having histological characteristics similar to CCP or gastritis cystica profunda, can be found in any part of the gastrointestinal tract7 and one found in the small intestine is called ECP.
With regard to the cause of ECP, there is the theory that if epithelial cells settle on the submucosal layer after an ulcer of mucous membrane or the inflammatory destruction of the submucosal layer, the cystic dilatation of gland takes place as a process to repair the damage,3,8 and another theory that the disease occurs in neonates due to chromosomal aberration accompanied by various congenital diseases.1,3
Most of the cases reported so far had accompanying diseases, and the most common accompanying diseases were Crohn's disease and Peutz-Jeghers syndrome. Karnak and colleagues3 reported that among 11 cases of ECP reported in the past, six cases were accompanied by Crohn's disease, three by Peutz-Jeghers syndrome, one by hamartomatous polyp, and one by multiple congenital anomaly. According to the report, the site of ECP was the ileum in seven cases, jejunum in three cases, and duodenum in one case.3
In Korea, three cases of ECP have been reported so far. One case was accompanied by Peutz-Jeghers syndrome and occurred in the jejunum,4 another was accompanied by gastric adenocarcinoma and occurred in the descending part of the duodenum,2 and the other occurred in the duodenal bulb without accompanying disease.5 However, there has been no case like ours that occurred in the ampulla of Vater without accompanying disease and was misdiagnosed as choledochocele.
ECP has various nonspecific clinical symptoms, and its symptoms can vary depending accompanying diseases. Most foreign cases showed symptoms such as diarrhea, abdominal pain, bleeding, and ileus depending on the accompanying disease,3,9 and some cases were diagnosed through surgical resection because of its acute abdominal picture despite the absence of accompanying disease10 but our case did not have any symptom.
ECP is diagnosed usually through upper gastrointestinal series, endoscopy, EUS, and abdominopelvic computed tomography, and can be diagnosed definitely by resected specimen using surgical operation or endoscopic polypectomy.2 ECP is observed in the form of cystic mass. In our case, a soft polypoid lesion covered with normal mucosa identical with surrounding mucosa was observed endoscopically in the proximal part of the major papilla of the ampulla of Vater. When pressed with biopsy forceps, it sank smoothly, showing a positive pillow sign. So we suspected it as choledochocele, a kind of bile duct cyst, and performed MRCP and ERCP.
Histopathologically, ECP is believed to occur when the tissue of hyperplastic mucous membrane goes over the natural barrier and invades into the basal tissue due to the destruction of lamina muscularis mucosa. A large lesion has the irregular proliferation of the mucous gland, is usually cystic and limited under the mucous membrane but rarely it can extend to the serous membrane.11,12 ECP should be differentiated from diseases such as acquired diverticula, pneumatosis cystoides intestinalis, and most importantly, mucinous adenocarcinoma. Characteristic histological findings of ECP for differentiation are irregular distribution of glands and cysts, the lack of desmoplastic stroma, the presence of surrounding lamina propria, the absence of cytologic abnormalities in the lining epithelium, the differentiation of signet cells from muciphages, and the lack of floating atypical epithelial fragments within pools of mucin.1 In this case, multiple cystic structure lined with mucous columnar epithelia was observed in the submucosal layer, and epithelial cells in the mucous membrane did not show any atypical or malignant change, so it could be diagnosed as ECP.
ECP does not require any special treatment, but occasionally, surgical resection or endoscopic polypectomy can be performed in order to treat symptoms such as obstruction or bleeding or for a diagnostic purpose to exclude malignant tumor, for which currently endoscopic polypectomy is common.2
The authors experienced a case of ECP in a healthy patient without any particular accompanying disease who had a submucosal tumor in the ampulla of Vater found by accident, which was suspected as choledochocele at first but was histopathologically diagnosed as ECP through endoscopic polypectomy, so report the case here.