INTRODUCTION
Since 1991 when Dohmoto used self-expandable metal stent (SEMS) for obstruction due to rectal cancer, SEMS has been widely used for obstructive colon cancer.1
Colon obstruction due to colon cancer occurs in about 30% of colon cancer patients as a common cause of emergency surgery;2,3 however, emergency surgery in these patients leads to complications in about 60% and even death in up to 22% of the patients,4 and transient or permanent colostomy might be developed after surgical intervention, causing declined quality of life of the patient.5 Approximately 30% of colon cancer patients with colon obstruction accompanies extensive tumor invasion, metastasis and underlying disease, making them unsuitable for curative surgery.6
In patients with colon obstruction due to colon cancer, stenting is an effective strategy to decompress the colon and is helpful in decreasing the risk of emergency surgery and buying some time to improve the patient's poor condition before the surgery. Stenting allows performing necessary tests before the surgery to evaluate the extent of invasion and the presence of metastasis accurately. It also leads to bowel preparation enabling elective surgery. Patients with multiple metastases that were found unresectable or with high risk for operation may avoid unnecessary surgery or colostomy because stent insertion itself may work as a palliative treatment.6,7 Despite these merits, however, stent insertion may cause various complications including bowel perforation, stent migration, restenosis, and bleeding.8
The most serious form of complication is colon perforation. Sebastian et al.9 reported colon perforation in 3.7% of patients who received SEMS insertion due to colon cancer, stating balloon dilatation before the stent insertion as the cause of the perforation. There have been reports that a misplaced stent in a curved lesion is associated with perforation;10,11 however, there are few literature so far on contraindications or the angle of stenotic lesion associated with the perforation, although there have been reports on various causes of perforation when using SEMS. The authors analyzed and report here the factors associated with perforation in colon cancer patients who received SEMS insertion.
MATERIALS AND METHODS
We performed a retrospective review on colon cancer patients who received SEMS insertion as a palliative treatment or a bridge to curative surgery for colon obstruction at CHA Bundang Medical Center, CHA University between January 2002 and August 2011. The Institutional Review Board of the CHA Bundang Medical Center, CHA University approved this study (IRB no. BD2012-146D). Data on the patients' baseline characteristics, site and stage of tumors, the purpose of stenting, type and length of the stent, the length and angle of stenosis, and attending physicians were obtained from chart review to determine the association with perforation. Niti-S stent (Taewoong Co., Ltd., Seoul, Korea) and Hanaro stent (MI Tech Co., Ltd., Seoul, Korea) were used as the SEMS for the procedure. Under the fluoroscopic guidance, the stent delivery system was advanced directly after passing the guide-wire through the lesion or by using through the scope endoscopy. To visualize best view of the lesion, we had the patients turned around under fluoroscopy. Stenosis angle was measured by using the fluoroscopic images taken at the time of SEMS insertion. We chose best image among the films. Angle was measured with the axes of both ends from angulated lesion by guide wire or insertion sheath of SEMS with picture archiving and communication system (Marosis m-view 5.0; Marotech Inc., Seoul, Korea) measuring program. After SEMS insertion, this angle becomes blunter due to straightening power of the stent. SPSS version 19.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis, with p-value of less than 0.05 as significant. Student t-test, chi-square test, Fisher exact test, and logistic regression were used for the analysis.
RESULTS
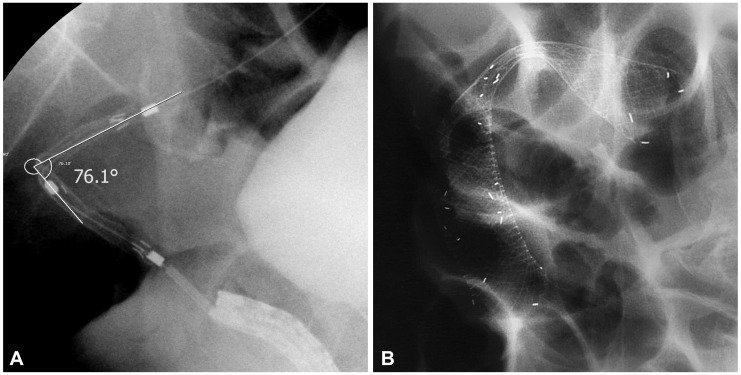
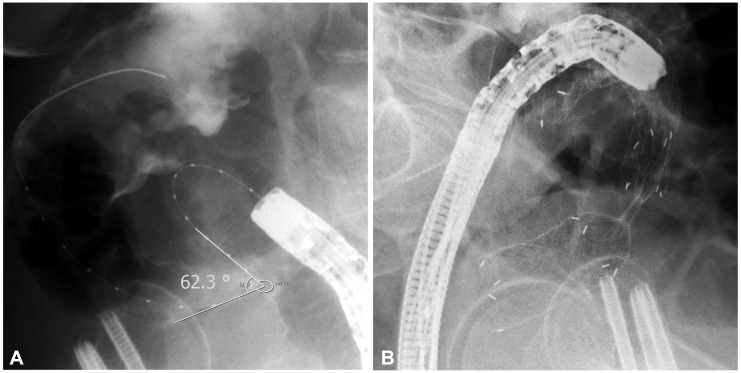
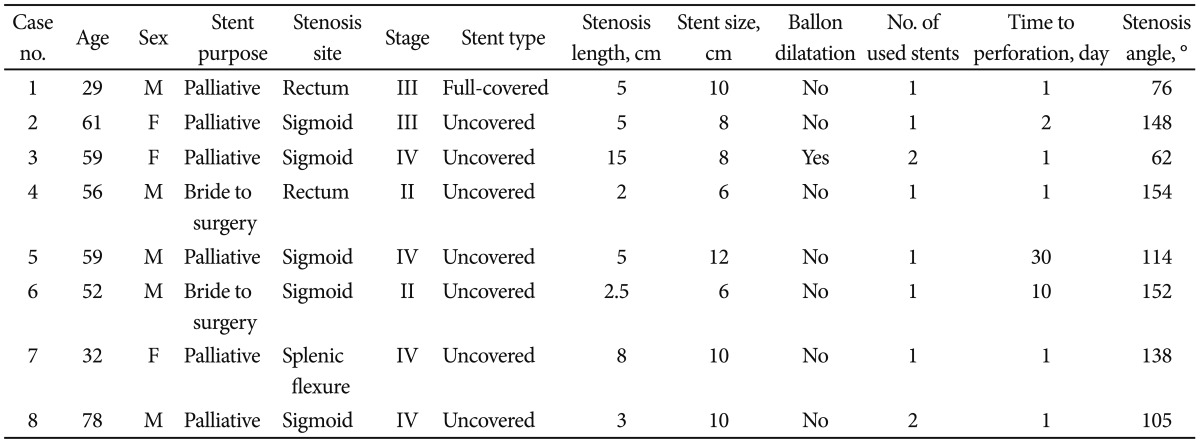
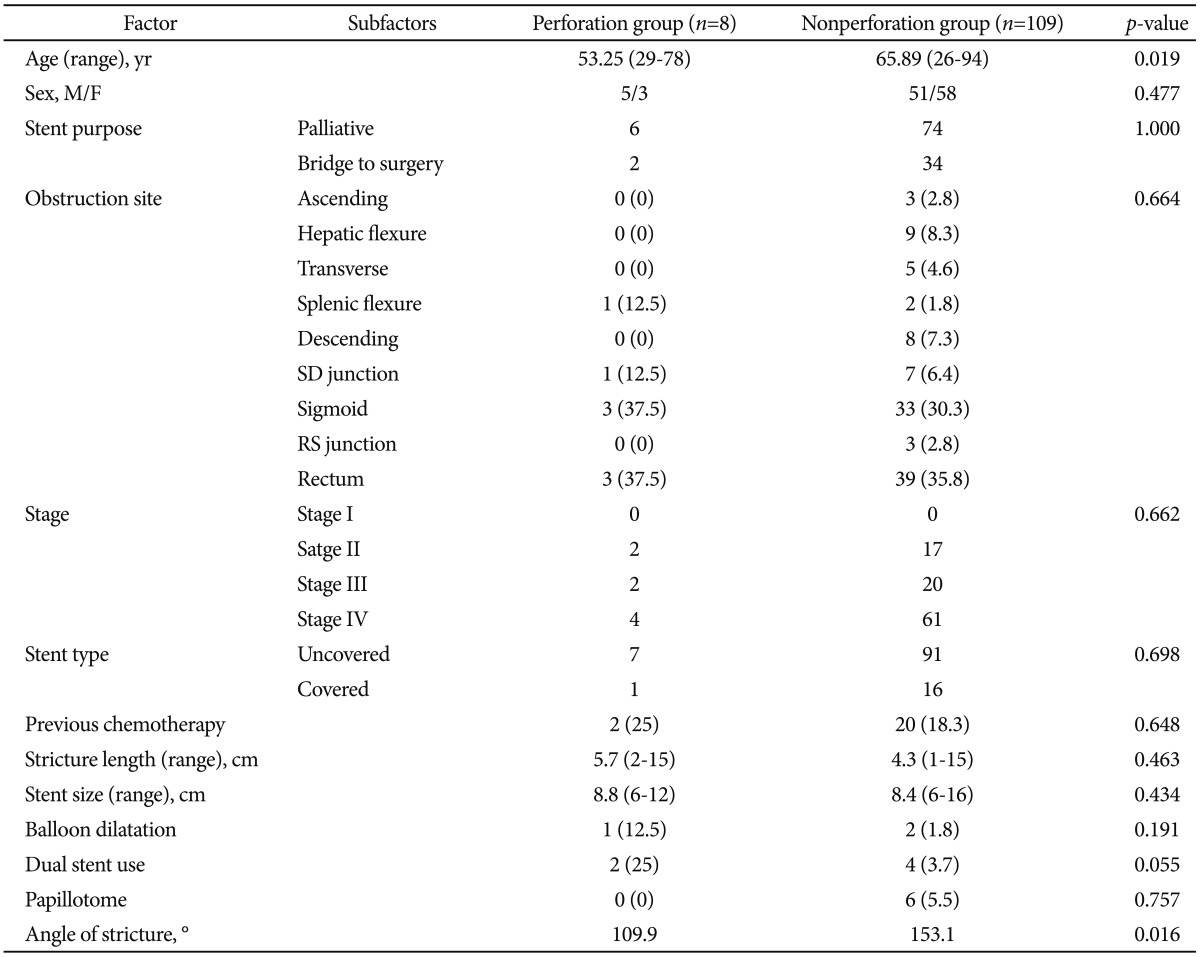
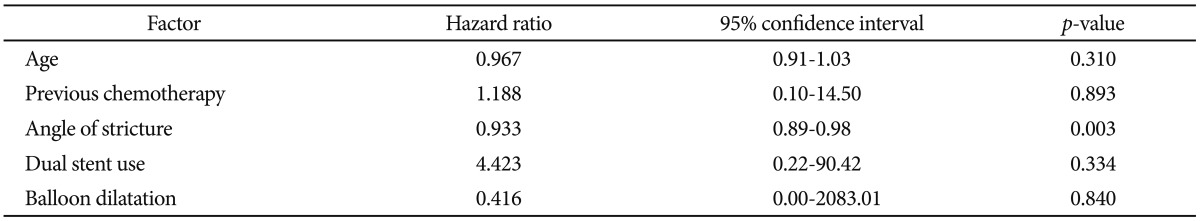
SEMS insertion was performed in 130 cases of obstructed colon cancer at our institution but 13 cases were excluded from the analysis due to poor fluoroscopic images. Excluded cases were all nonperforated cases. Finally, 117 cases were evaluated. Perforation occurred in eight patients of them (Table 1). Six of them were for palliative purpose and two were as a bridge to surgery. Among the eight patients with perforation, five patients had stenotic tumor in the sigmoid colon, two in the rectum, and one in the splenic flexure. Colon cancer stage was IV in four out of the eight patients, but the frequency was not different from nonperforation group. Uncovered stent was used in all patients, except for one patient who received full-covered stent. The median time from the stent insertion to perforation was 1 day (range, 1 to 30 days). Among the eight patients with perforation, the mean stenosis angle was 109.9┬░ compared to 153.1┬░ in the nonperforation group, indicating that the angle was more acute in the perforation group (p=0.016). Figs. 1, 2 show fluoroscopic images of perforated cases after SEMS placement at the angular stenotic lesion. The perforation occurred at the splenic flexure and sigmoido-descending colon junction only in one patient each, and at the rectum or sigmoid colon in six patients; however, rectosigmoid colon obstruction accounted for 75% of the nonperforation group, suggesting that the difference between the groups was not statistically significant. Three patients received balloon dilatation, and one of them experienced perforation. Six patients received multiple stents of two, and two of them experienced perforation. The lengths of the stents and stenosis were not different between the groups. The clinical and technical characteristics of the two groups are summarized in Table 2 and multivariate analysis of risk factors of perforation is shown in Table 3. Physicians who performed stent insertion were all experts of the procedure with over 10 years of experience. Perforation rate was not different among physicians (p=0.4).
DISCUSSION
The incidence of bowel perforation in this study was 6.2% (8/130), which was higher than the incidence (3.7%) reported by Sebastian et al.9 Bowel perforation might occur firstly while inserting guide-wire or catheter due to excessive manipulation, in which case the perforation can be confirmed by the leakage of the contrast dye through the abdominal cavity.12,13 Also the endoscopist's experience may be a factor since less-experienced endoscopists tend to perform excessive manipulation more often.14 All the endoscopists in our institution have more than 10 years of experience in endoscopy. Second, balloon dilatation may cause perforation, which is why balloon dilatation is not recommended before stent insertion.6 Khot et al.6 reported that perforation occurred in about 2% of the patients in the group without balloon dilatation, compared to 10% in the group with balloon dilatation, and insisted that a thin stent delivery device that could pass through the stenosis might reduce the need for balloon dilatation and perforation as a result. In our study, balloon dilatation was performed in only three patients among the overall 117 patients, and one of them experienced perforation. Excessive air inflation during the procedure might be associated with perforation.9 Third, friable tumor tissues may cause perforation due to strong radial force during the stent expansion.15 Fourth, colonic wall erosion due to the stent ends may be the cause of perforation; most of these cases are microperforation without symptoms and the inflammatory reaction of the surrounding tissues are not serious.15 These cases are typically presented as delayed perforation, and such erosion occurs often at the ascending colon, which has thinner colonic wall than the rectum. This is also why the perforation occurs more often at the right colon than the left colon.16 According to a study report, a rigid type Wall-stent (Boston Scientific, Natick, MA, USA) was found to cause erosion and ulcer more often compared to a flexible type Ultraflex (Boston Scientific), thereby increasing the risk of perforation.17
We compared stenosis angle and various characteristics between the perforation group and the nonperforation group while analyzing the patients with perforation after SEMS insertion. First of all, stenosis angles of the perforation group and the nonperforation group were 109.9┬░ and 153.1┬░, respectively, with statistically significant difference (p=0,016). Perforation occurred in five cases among the seven cases with less than 120┬░ of stenosis angle in particular, strongly suggesting that the perforation increases as the stenosis angle becomes more acute. This can be explained by the possibility of injury during the manipulation of a guide-wire or a stent delivery system, excessive tensile force to the outer corner wall during the stent expansion, and erosion and ischemia from the pressure to the colon wall caused by a long stent ends leading to a perforation. Lastly, very angular stricture needs multiple stents due to the difficulty of complete stent insertion, which means that perforation might be increased due to the greater pressure at the sites where the stents are overlapped or at the end of the stents. Song et al.10 reported increased perforation when using a dual-design stent. In our study, two stents were used in six cases, and perforation occurred in two cases, reflecting relatively more frequent use of a dual stent in the perforation group. Song et al.10 used a dual-design stent for every case from the start, whereas we used dual stents only for the cases with obstructions that cannot be fully relieved with one stent, suggesting the possibility that not only the use of a dual stent but also the characteristics of the stricture itself contributed to the occurrence of perforation. The two patients who experienced perforation after using a dual stent actually had stenosis angles of 62┬░ and 105┬░, which were significantly more acute than the average stenosis angle of the four patients without perforation (154┬░). Based on these results, it would be advisable to use a flexible stent with less dynamic stability, not to perform balloon dilatation before and after the stent insertion, and to avoid using multiple stents if possible, in order to prevent perforation when inserting SEMS to an angular stenosis.
Although perforations occurred often at curved lesions, the frequency was not statistically different from that of the nonperforation group, suggesting that the angle and characteristics of the stenosis, not specific location of the lesion, are important factors.
Bowel status of preparation was similarly poor because all indicated cases were inappropriate to prepare due to obstruction. Retrospective study was another reason for evaluation failure about preparation.
One of the limitations of this study is the fact that the stenosis angle was measured retrospectively based on the fluoroscopy images, and that several cases had to be excluded from the analysis due to the inaccuracy of the image. Prospective studies with stenosis angle measurement might enable more precise analysis based on the correlation between the stenosis angle and perforation observed in this study. Secondly, we could not identify the exact location of the perforations. The location of perforation changes depending on whether the radial force of stent caused tear in the stricture or the stent ends caused erosion-and perforation as a result-by touching the normal lumen. It would be possible to find the major cause of perforation if the exact location of perforation could be identified by observing resected colon specimen in the future.
In conclusion, our institutional experience suggests that angular positioning of stent increases bowel perforation after SEMS placement for malignant colorectal obstruction. It is advisable, therefore, to pay special attention on the possibility of a lumen injury when manipulating a guide-wire or a stent delivery system, to use a more flexible stent so as to reduce the tensile force, to avoid using balloon dilatation before and after the stent insertion, and to avoid using multiple stents if possible, for an angular stenosis lesion.