INTRODUCTION
Since superficial nonampullary duodenal epithelial tumors (SNADETs) have a lower prevalence compared to gastrointestinal tumors, specific clinical practice guidelines have not yet been established in Japan. Recently, the incidence of SNADETs has been increasing due to the widespread use and development of endoscopy. Many treatment options are available for SNADETs, such as conventional endoscopic mucosal resection (cEMR), endoscopic submucosal dissection (ESD), laparoscopic and endoscopic cooperative surgery for duodenal tumors (D-LECS), and surgical resection including pancreatoduodenectomy. Recently, cold snare polypectomy (CSP) and underwater EMR (UEMR) have been reported as minimally invasive procedures for SNADETs, but the treatment strategies have not yet been standardized. We reviewed the treatment methods for SNADETs and presented the treatment outcomes of CSP and UEMR.
TREATMENTS FOR SNADETs
Natural history and follow-up with repeated biopsy
In addition to the colorectum, adenoma-adenocarcinoma sequences have been reported in the duodenum [1]. Okada et al. [2] reported that approximately 25.9% (11/43) of low-grade adenomas grew to high-grade dysplasia or noninvasive carcinomas over 6 months. However, the diagnostic accuracy of biopsy is unreliable, and biopsy scars render endoscopic resection more difficult. Kinoshita et al. [3] reported that the histopathological diagnostic capability of a biopsy for duodenal carcinoma had a sensitivity of 37.5%, a specificity of 83.1%, an accuracy of 71.6%, and a positive predictive value of 79.7%. Additionally, 15 of 61 patients (24.6%) with a positive non-lifting sign and treatment modality were converted from EMR to ESD due to unexpected fibrosis from a preoperative biopsy. Endoscopic resection is recommended if the lesion can be safely resected because there is lacking information on its progression. We do not recommend follow-up with repeated biopsies of SNADETs.
cEMR
Using cEMR for 240 SNADET lesions measuring less than 20 mm, Kiguchi et al. [4] reported en bloc and R0 resection rates of 96% and 80%, respectively. Regarding adverse events, no perforation was reported, and 1% (1/240) of lesions developed delayed bleeding. Among the lesions larger than 20 mm, the en bloc resection rate of cEMR was reported to be 30%ŌĆō40% [5]. Conversely, Nonaka et al. [6] reported the long-term outcomes of cEMR cases including piecemeal resection cases. Although the rate of en bloc resection was only 64%, no residual recurrence was observed during the median follow-up period of 51 months. However, it has been reported that the recurrence rate after cEMR for adenomas larger than 20 mm was 30.1% (22/73), and increasing lesion size was a significant risk factor for recurrence [7].
The indication of cEMR for SNADETs should be limited to lesions smaller than 20 mm in size. However, for lesions larger than 20 mm in size, piecemeal EMR may be a treatment option if an appropriate lesion can be selected.
CSP
As mentioned above, small duodenal adenomas are often diagnosed through biopsies. However, the diagnostic accuracy of a biopsy for duodenal carcinoma is unreliable, and repeated biopsy makes endoscopic resection more challenging. Therefore, instead of a biopsy, we believe that the duodenal adenoma should be removed when it has a small size if it can be performed safely and easily.
CSP has become a standard procedure for small colorectal adenoma lesions smaller than 10 mm in size. CSP is a simple and quick procedure that does not involve submucosal injection or electrocautery and has the same complete resection rate and bleeding rate as of hot snare polypectomy [8]. We considered CSP to be suitable for small duodenal lesions and it had been introduced for SNADETs in 2015 at our institution [9].
Maruoka et al. [10] reported the safety and efficacy of cold polypectomy (CP, CSP, and cold forceps polypectomy) for sporadic SNADETs. Thirty lesions in 22 patients were resected using CSP, and the median lesion size was 4 mm (range, 2ŌĆō6 mm). The en bloc and R0 resection rates were 96.7% and 68.0%, respectively, and no intra- or delayed bleeding was observed. Follow-up endoscopy was performed in all patients 3 months after CSP, and no recurrence was observed (Table 1). Hamada et al. [11] also investigated CSP for small multiple duodenal adenomas in patients with familial adenomatous polyposis. Twenty-six lesions in 4 patients were resected by CSP with no delayed bleeding, although the defect after CSP was not closed with endoclips in all patients.
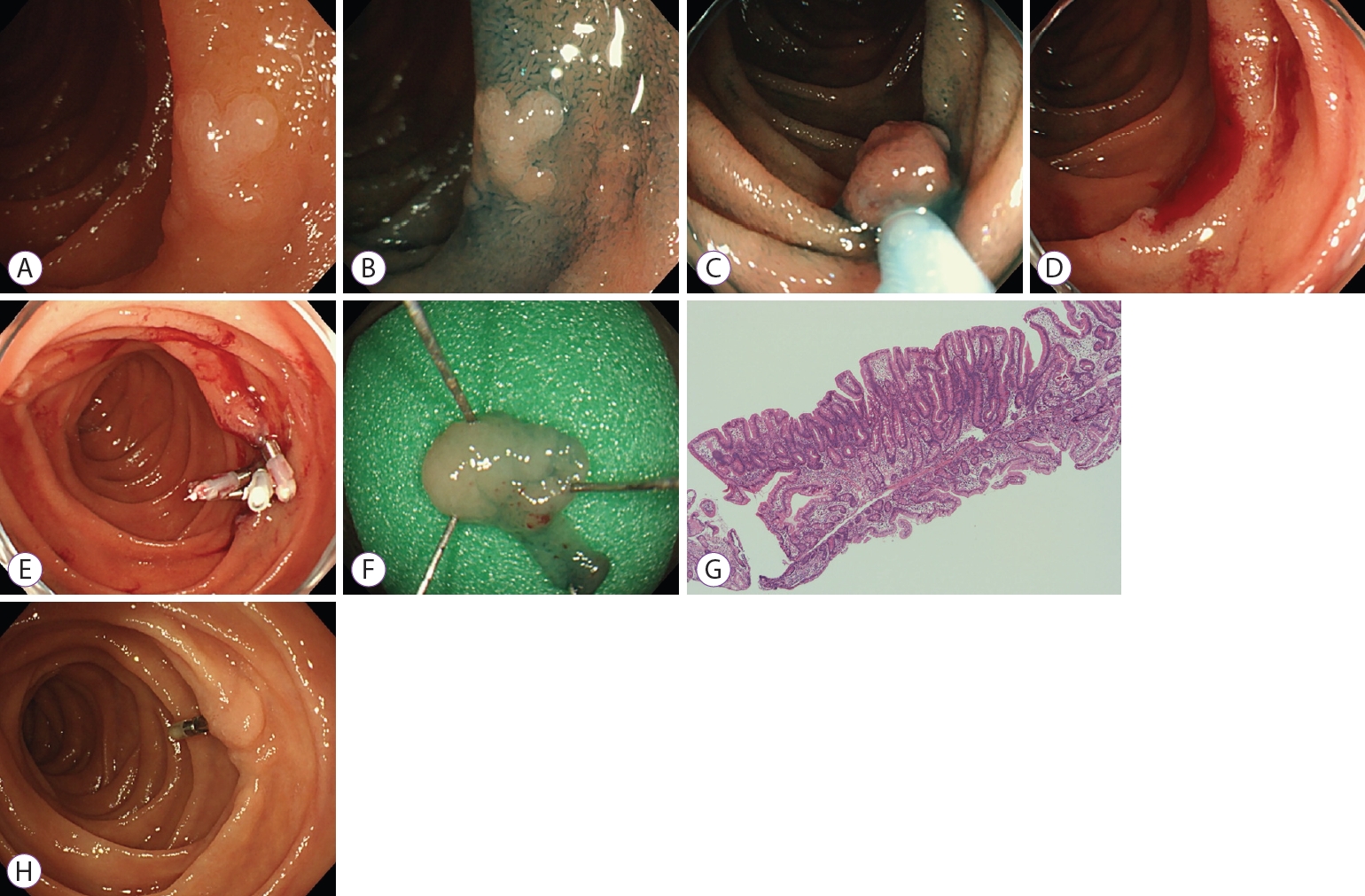
At our institution, CSP is indicated for SNADETs for the endoscopic diagnosis of tubular adenomas that are 10 mm or smaller in size (Fig. 1). A preoperative biopsy scar is not always a contraindication for CSP. Fifty-three lesions in 47 patients (male/female: 37/10; median age: 67 years [range: 39ŌĆō82 years]) were resected by CSP from January 2015 to July 2020 at the Shizuoka Cancer Center. As for the lesion location, six were in the 1st portion, 45 in the 2nd portion, and two in the 3rd portion. The median lesion size was 6 mm (range, 2ŌĆō12 mm). In the Paris classification, the macroscopic type was 0-I in 6 lesions, 0-IIa in 43 lesions, 0-IIa+IIc in 1 lesion, and 0-IIc in 3 lesions. Biopsy before CSP was performed in 25 lesions (47.2%). During this study period, CSP was attempted for 53 lesions, but 6 lesions could not be resected without electrocautery. Thus, these were removed by cEMR with electrocautery, with a CSP complete rate of 88.7%. The median size of the resected specimen was 11.5 mm (range: 5ŌĆō25 mm), the rates of en bloc and R0 resection were 96.2% (51/53) and 45.7%, respectively. The median procedure time (from the end of observation to the completion of resection) was 3.5 min (range: 1ŌĆō23 min), and the rate of spurting bleeding immediately after CSP was 0%. After CSP, the ulcer was closed with endoclips in 31 lesions (57%). No adverse events and delayed complications (bleeding or perforation) were observed during the procedure. Histopathological assessments revealed 42 adenomas, 3 adenocarcinomas (intramucosal), and 8 non-neoplastic lesions. Among the 45 neoplastic lesions (adenoma and adenocarcinoma), the horizontal margins (HMs) were negative in 21, positive in 1, and undetermined in 23. Moreover, the vertical margins (VMs) were negative in 41, positive in 1, and undetermined in 3. At our institution, 47 of 53 lesions were followed up for more than one month using an endoscope. During the median observation period of 19 months (range: 1ŌĆō52 months), only one residual recurrence (2.1%) was reported, which could be treated by cold forceps polypectomy (CFP).
Based on our results, CSP might be useful for duodenal adenoma lesions smaller than 10 mm in size. The efficacy of CSP should be evaluated based on the long-term outcomes in a multicenter prospective study.
UEMR
Binmoeller et al. [12,13] first reported UEMR for colorectal polyps in 2012 and then for duodenal adenoma in 2013. Superficial lesions float up like protruded lesions with water immersion, allowing them to be easily snared and removed, even for flat or sessile lesions.
Iwagami et al. [14] reported the technical outcomes and follow-up data of UEMR for SNADETs. The en bloc resection rate was 79% for lesions <20 mm and 14% for lesions Ōēź20 mm. The delayed bleeding rate was 2%, and the delayed perforation rate was 0.6%. The recurrence rate after UEMR was 4.5% (7/157), but all residual lesions were retreated with additional endoscopic resection (Table 2).
In general, UEMR for SNADETs is performed as follows. After observing the lesions by white light imaging and narrow band imaging, the gastric and duodenal air should be deflated, and saline solution should be injected through the water jet. After filling the lumen with water, resection with electrocautery was performed while ensuring that the lesion was completely snared. Then, the specimen was grasped with forceps and removed with the scope, because aspiration may cause the specimen to piecemeal. Please refer to references for detailed procedural information [15].
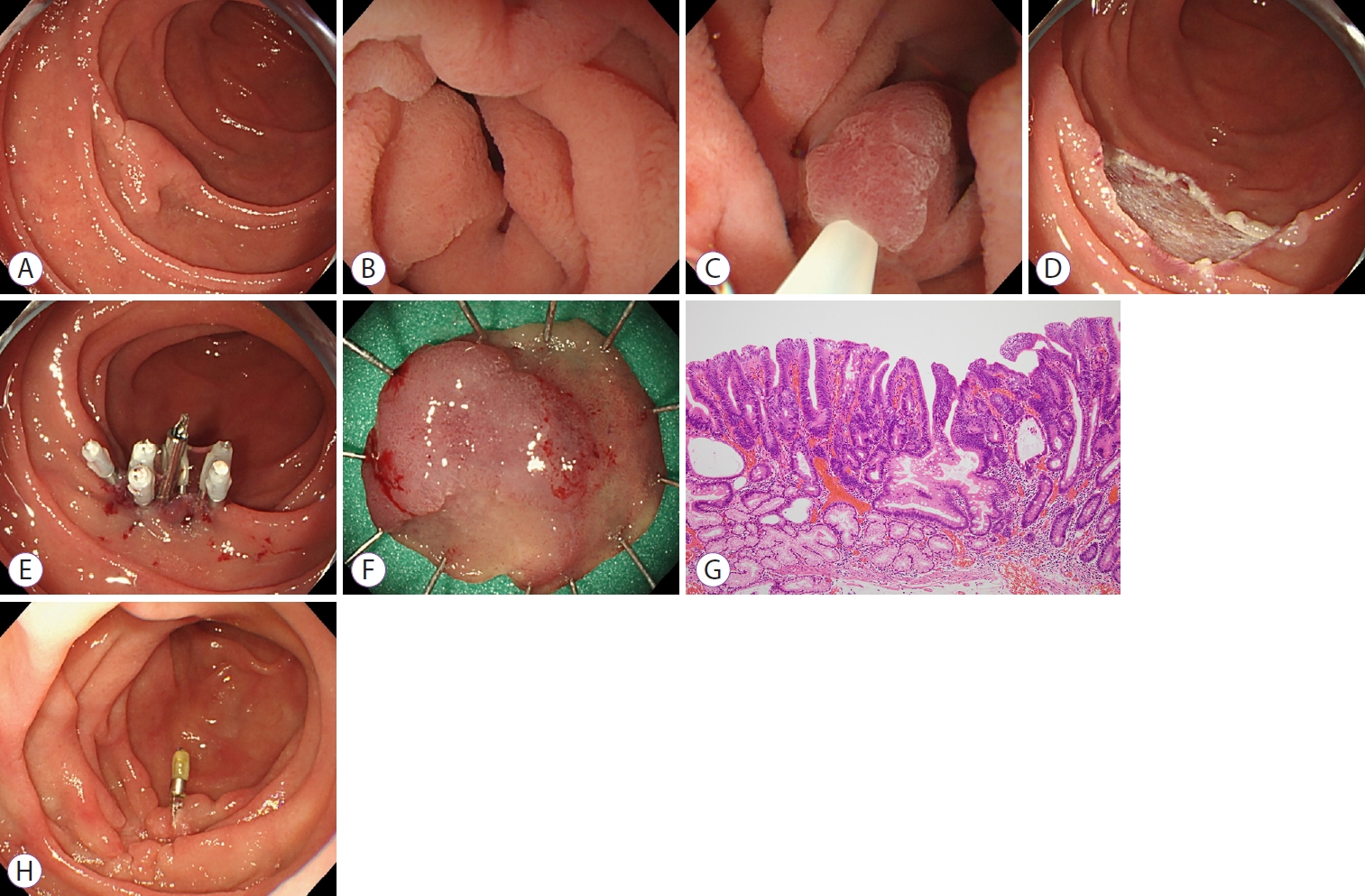
At our institution, UEMR for SNADETs is indicated to endoscopically diagnose adenomas larger than 10 mm or intramucosal carcinomas no larger than 30 mm in size (Fig. 2). In total, 65 lesions in 54 patients (male/female: 31/23; median age: 67 years [range, 28ŌĆō89 years]) were resected by UEMR from January 2015 to July 2020 at the Shizuoka Cancer Center. The lesion was located in the 1st portion in 9, in the 2nd portion in 52, and in the 3rd portion in four lesions. The median lesion size was 12 mm (range, 3ŌĆō25 mm). In the Paris classification, the macroscopic type was 0-I in 8 lesions, 0-IIa in 36 lesions, 0-IIa+IIc in 17 lesions, and 0-IIc in 4 lesions. A biopsy before UEMR was performed on 26 lesions (40%). During the study period, all the lesions that had undergone UEMR were resected without conversion to any other procedure such as ESD (the UEMR complete rate was 100%). The median procedure time (from the completion of water immersion to the end of resection) was 5 min (range: 1ŌĆō104 min). The rate of spurting bleeding immediately after UEMR was 0%. Furthermore, after UEMR, the ulcer was closed using endoclips in 59 lesions (91%). The median resected specimen size was 15 mm (range: 6ŌĆō40 mm), and the rate of en bloc resection was 86% (56/65). No adverse events were observed during the procedure. Delayed bleeding occurred in one lesion (1.5%), and no delayed perforation occurred after UEMR. Histopathological assessments revealed 46 adenomas, 15 adenocarcinomas (intramucosal), and four non-neoplastic lesions. Among the 61 neoplastic lesions (adenoma and adenocarcinoma), the HMs were negative in 32, positive in one, and undetermined in 28. As for the VMs, 59 were negative, one was positive, and one was undetermined. At our institution, 48 of 65 lesions were followed up for more than one month using an endoscope. During a median observation period of 4 months (range: 1ŌĆō29 months), local recurrence was observed in two of 48 lesions (4%) and were treated using re-UEMR. Although the number of HMs-negative cases was less than 50%, the residual recurrence rate was less than 4%, but all recurrent lesions could be resected endoscopically. We concluded that a duodenal adenoma larger than 10 mm and an intramucosal carcinoma equal to or smaller than 30 mm could be a good indication for UEMR. Further examination in a prospective study with a longer follow-up period is necessary to confirm the efficacy and safety of UEMR.
ESD
ESD is the standard endoscopic resection procedure for tumors in the esophagus, stomach, and colon. However, for SNADETs, ESD has not been widely accepted as a standard treatment because of the high complication rates, primarily due to the thin duodenal wall, poor maneuverability of the scope, and exposure to bile and pancreatic juice [16]. Based on the results of 49 patients who underwent ESD for SNADETs larger than 20 mm, Hoteya et al. [17] reported an en bloc resection rate of 98% and an R0 resection rate of 84%. In this report, no local recurrence or distant metastasis was observed during the median follow-up period of 76 months. Yahagi et al. [18] also reported remarkable treatment outcomes of ESD for SNADETs, with en bloc and R0 resection rates of 98.3% (171/174) and 85.1% (148/174), respectively. However, the complication rates were still high (5.2% for delayed bleeding and 15.5% for perforation). Recently, they reported encouraging results for the newly developed procedure, known as the ŌĆ£water pressure method,ŌĆØ for duodenal ESD [19]. However, duodenal ESD is a highly complex endoscopic technique that requires extensive experience. Therefore, we consider that ESD should only be performed at high-volume centers, and the lesion should be carefully selected.
Prophylactic endoscopic closure after ER
The rate of intraoperative perforation in duodenal endoscopic procedures is higher than that in other gastrointestinal lesions. However, postoperative complications must be considered, particularly postoperative perforation, which is a risk factor for emergent surgery. This is mainly caused by the exposure of the ulcer after endoscopic resection of pancreatic juice and bile.
Kato et al. [20] reported the need for complete closure at the post-ESD site in 168 patients (173 lesions) who had undergone duodenal ESD. The delayed perforation and bleeding rates in the complete closure group vs. the incomplete/unclosed group were 1.7% vs. 10.5% and 0% vs. 10.5%, respectively. The postESD site for SNADETs must be completely sutured to prevent delayed complications, though a single reliable and safe method has not yet been recommended because various closure methods have been reported. Prophylactic defect closure by clips is the most standard method, but it is insufficient for large post-ESD ulcers. Therefore, suturing methods using over-the-scope clips or various tractions are used as needed [21-25]. As an alternative to suturing, polyglycolic acid sheets and fibrin glue can be useful [26]. It may be possible to reduce the occurrence of complications due to premature dislodgement of the prophylactic clips.
D-LECS
LECS was developed for gastric submucosal tumors [27], but it has also been utilized for duodenal neoplasms. This relatively new procedure is less invasive than conventional surgery [28]. D-LECS is roughly divided into two types depending on whether a temporary perforation is performed during the endoscopic procedure: full-thickness resection and ESD with laparoscopic reinforcement. This technique is believed to compensate for the high perforation rate in ESD with reinforcement using seromuscular sutures at the mucosal defect site. Nunobe et al. [29] showed that the rates of en bloc and R0 resection during D-LECS were 96% (198/206) and 95% (196/206), respectively. In this study, D-LECS had the same indications as ESD in duodenal tumors, for example, adenoma and mucosal adenocarcinoma without around the ampulla of Vater. Additionally, submucosal tumors, such as gastrointestinal stromal tumors and neuroendocrine tumors, were also observed. Intraoperative and delayed perforations occurred in 4.3% (9/206) and 2.4% (5/206) of lesions, respectively. The Clavien-Dindo classification grade Ōēź3 postoperative complication rate was 4.4% (9/206), and the postoperative perforation rate was 1.5% (3/206). We should be aware that the delayed perforation rate is not 0%, even if the mucosal defect is laparoscopically reinforced using seromuscular sutures.
CONCLUSIONS
Because of the higher complication rates of ESD for SNADETs compared to other digestive tract tumors, it is challenging to select a standard procedure similar to that for esophageal, gastric, and colorectal intramucosal carcinomas. The proposed treatment strategy for SNADET is shown in Fig. 3. A duodenal adenoma 10 mm or smaller in size is an indication for CSP or follow-up without biopsy, while those larger than 10 mm or lesions of suspected intramucosal carcinoma are indicated for UEMR. For lesions larger than 30 mm, the treatment method can be selected among various methods, such as surgical resection, ESD, hybrid ESD, and D-LECS. The treatment plan should be selected on a case-to-case basis, considering the balance between the risk of complications and the necessity of en bloc resection.