A novel fully covered metal stent for unresectable malignant distal biliary obstruction: results of a multicenter prospective study
Article information
Abstract
Background/Aims
Endoscopic self-expandable metal stent (SEMS) placement is currently the standard technique for treating unresectable malignant distal biliary obstructions (MDBO). Therefore, covered SEMS with longer stent patency and fewer migrations are required. This study aimed to assess the clinical performance of a novel, fully covered SEMS for unresectable MDBO.
Methods
This was a multicenter single-arm prospective study. The primary outcome was a non-obstruction rate at 6 months. The secondary outcomes were overall survival (OS), recurrent biliary obstruction (RBO), time to RBO (TRBO), technical and clinical success, and adverse events.
Results
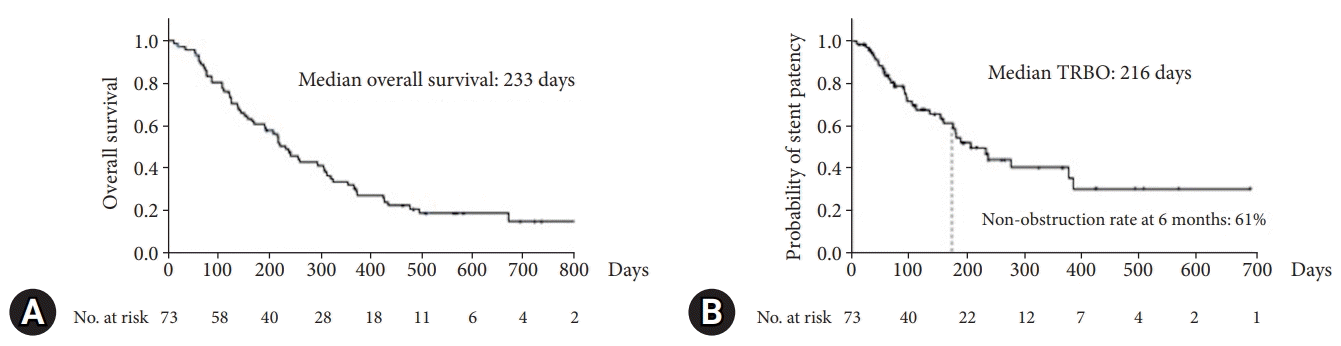
A total of 73 patients were enrolled in this study. The non-obstruction rate at 6 months was 61%. The median OS and TRBO were 233 and 216 days, respectively. The technical and clinical success rates were 100% and 97%, respectively. Furthermore, the rate of occurrence of RBO and adverse events was 49% and 21%, respectively. The length of bile duct stenosis (<2.2 cm) was the only significant risk factor for stent migration.
Conclusions
The non-obstruction rate of a novel fully covered SEMS for MDBO is comparable to that reported earlier but shorter than expected. Short bile duct stenosis is a significant risk factor for stent migration.
INTRODUCTION
Unresectable malignant distal biliary obstruction (MDBO) results in obstructive jaundice that requires biliary drainage not only to improve the quality of life but also to achieve a drainage effect before the induction of chemotherapy. Currently, endoscopic transpapillary biliary drainage is the standard procedure for treating unresectable MDBO.1 Clinical guidelines recommend self-expandable metal stents (SEMSs) because they have a lower risk of stent dysfunction compared with plastic stents. Recent advances in anticancer treatment have improved the prognosis of patients with unresectable MDBO; therefore, longer stent patency is required.
The selection of covered or uncovered SEMSs for MDBO is debatable. Uncovered SEMSs pose the risk of stent occlusion following tumor ingrowth into the metal mesh.2 Covered SEMSs have been developed to resolve this problem. However, covered SEMSs are associated with a lower risk of tumor ingrowth, but a higher risk of stent migration. A recent systematic review and meta-analysis comparing the performances of covered and uncovered SEMSs in patients with unresectable MDBO concluded that the data showed no added benefits of covered SEMSs.3 However, based on the results of randomized controlled trials conducted in Japan, the patency of covered SEMSs is superior to that of uncovered SEMSs, with comparative adverse events.4,5 Therefore, covered SEMSs are mainly used in Japan, and covered SEMSs with longer stent patency and fewer stent migrations are strongly needed.
The fully covered SEMS (Cook Evolution Biliary Stent; Cook Ireland Ltd.) used in this study was constructed from a single woven cross-structured nitinol wire with a silicone-covered membrane. These stents have three potential advantages. First, although a low radial force (RF) is considered a risk factor for stent migration,6 this stent has a relatively high RF, and an appropriate axial force (AF) is expected to reduce stent migration. Secondly, the stent was flanged at both ends to reduce the risk of migration (Fig. 1A). Third, the stent is placed using a controlled-release, trigger-driven delivery system (Fig. 1B). This system allows the SEMS to be recaptured before stent placement, in case repositioning is necessary.7 This system also makes it possible to deploy the stent in an appropriate position; consequently, it is expected to reduce stent migration.

The fully covered self-expandable metal stent (SEMS) used in this study was constructed using a single woven nitinol wire with a silicone-covered membrane. The stent had flanged ends to mitigate migration (A). The stent was deployed using a controlled-release, trigger-driven delivery system. This system allowed recapturing of the SEMS before complete deployment if repositioning was required (B).
Therefore, we conducted a single-arm prospective study to determine the clinical efficacy of a novel, fully covered SEMS for treating unresectable MDBO.
METHODS
Study design
This multicenter, single-arm, prospective study was conducted at Kobe University, Japan. The patients were recruited between March 2018 and August 2019 from 12 hospitals in Japan (Kobe University Hospital, Osaka Saiseikai Nakatsu Hospital, Akashi Medical Center, Kita-Harima Medical Center, Kobe Medical Center, Kakogawa Central Hospital, Hyogo Cancer Center, Konan Hospital, Rokko Island Hospital, Takatsuki Hospital, Shiso Municipal Hospital, and Kobe Red Cross Hospital). The inclusion criteria were as follows: (1) age ≥20 years with the ability to provide informed consent and (2) pathologically proven, unresectable MDBO. MDBO was defined as a biliary stricture located 2 cm from the bifurcation. The exclusion criteria were as follows: (1) jaundice or cholangitis that could not be controlled by biliary drainage; (2) severe dysfunction in other organs (American Society of Anesthesiologist physical status grade III or IV); (3) Eastern Cooperative Oncology Group performance status 4; (4) life expectancy of less than 3 months; (5) coexisting hilar biliary stenosis; and (6) surgically altered upper gastrointestinal anatomy, such as Billroth-I, Billroth-II, or Roux-en-Y reconstruction. Patients in whom biliary cannulation by endoscopic retrograde cholangiopancreatography was unsuccessful were excluded from the study. Medical records were reviewed from the date of inclusion in the study until February 2020.
Procedures
In all the patients, SEMS placement was performed under conscious sedation by an experienced endoscopist at 12 hospitals. SEMSs were placed after endoscopic sphincterotomy. All SEMSs were 10 mm in diameter, and their lengths (6 and 8 cm) were determined at the discretion of each hospital. The SEMSs covered the biliary stenosis, and the lower end was placed across the papilla of Vater. It was unclear whether the patient had been treated with a plastic stent or other biliary drainage procedures prior to SEMS placement.
Outcomes
The primary outcome was a non-obstruction rate at 6 months. The secondary outcomes were overall survival (OS), recurrent biliary obstruction (RBO), time to RBO (TRBO), technical and clinical success, and adverse events.
The outcomes were defined according to the TOKYO criteria.8 RBO was defined as either stent occlusion accompanied by elevated liver enzyme levels and bile duct dilation on any image or symptomatic stent migration. The reason for stent occlusion was determined based on endoscopic or other imaging findings. TRBO was measured from the day of SEMS placement until RBO. TRBO was estimated using the Kaplan–Meier method. In the estimation, patient death and complications other than RBO requiring SEMS removal were treated as censored cases at the time of death and SEMS removal, respectively. Non-obstruction rates at 6 months were also estimated using the Kaplan–Meier method, where the non-obstruction rate is the rate of patients not subjected to RBO at 6 months. Technical success was defined as the successful placement of a SEMS with sufficient coverage of the stricture. Clinical success was defined as a reduction in serum bilirubin less than 1.3 mg/dL, or a decrease of ≥50% within 14 days. Pancreatitis, cholangitis, cholecystitis, perforation, bleeding, and stent migration were evaluated according to the TOKYO criteria. The length of the biliary strictures was measured using fluoroscopy.
Statistical analysis
In a previous study, the stent patency probability of a fully covered SEMS for MDBO at 6 months was 64%.9 Based on the results of this study, we hypothesized that the threshold for the stent non-obstruction rate at 6 months was 75%, and the expected stent patency probability at 6 months was 60%. Under these assumptions, the number of patients needed for this study was estimated to be 80, with a significance level of 0.05, a power of 0.8, and an α error of 0.05. Considering a withdrawal rate of 10%, the planned sample size was set at 90.
All statistical analyses were performed using JMP software version 11 (SAS Institute). All p-values were two-sided. Statistical significance was set at p<0.05. significant. Non-obstruction rates at 6 months, RBO, and OS were evaluated using the Kaplan–Meier analysis. RBOs were compared using log-rank tests. Risk factor analysis of stent migration was performed using the Cox proportional hazards model. Baseline variables (p<0.05) in the univariate analysis were included in the multivariate analysis.
Ethics statements
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of each hospital. All the patients provided written informed consent before participating in the study. This trial was registered with the University Hospital Medical Information Network (UMIN) under trial number 000030022.
RESULTS
Patient characteristics
The study period was repeatedly extended to reach the planned 90 patients; however, this was unsuccessful because of a higher rate of stent migration. According to the study protocol, case recruitment was discontinued upon the agreement of all participating hospitals, and recruitment was terminated in 81 patients. Eight of the 81 patients were excluded after study inclusion (four patients failed to undergo SEMS placement, two patients had coexisting hilar biliary stenosis that was noticed later, one patient had surgically altered anatomy that was noticed later, and one patient withdrew consent), and a total of 73 patients were enrolled in this study. Table 1 shows the baseline patient characteristics. The predominant cause of MDBO was pancreatic cancer (76.7%, 56/73). Prior biliary drainage was performed in 71.2% (52/73) of the cases. Prior cancer treatment, including chemotherapy or radiotherapy, was performed in 30.1% (22/73) of the cases, and cancer treatment after the procedure was performed in 57.5% (42/73) of the cases.
Technical success, clinical success, RBO, and adverse events
Table 2 shows the technical and clinical success, RBO, and the occurrence of adverse events. Technical success was achieved in 100% (73/73) of the cases. Clinical success was achieved in 97.3% (71/73) of patients. RBO occurred in 49.3% (36/73) of cases, and the causes of RBO were distal migration in 28.8% (21/73), proximal migration in 6.8% (5/73), sludge obstruction in 6.8% (5/73), overgrowth in 2.7% (2/73), and others in 4.1% (3/73). Adverse events occurred in 20.5% (15/73) of the cases, pancreatitis in 9.6% (7/73), cholecystitis in 8.2% (6/73), and non-occlusive cholangitis in 5.5% (4/73). No adverse events resulted in death. The median observational period was 217 days (interquartile range, 110–371 days).
Patient survival, time to stent patency, and TRBO
Figure 2 shows the Kaplan–Meier curves for patient survival and TRBO. The median OS was 233 days. The median TRBO score was 216. The non-obstruction rate at 6 months was 61%.
Risk factors for stent migration
The non-obstruction rate at 6 months was lower than expected, which could be due to the high frequency of stent migration. Therefore, we analyzed the risk factors for stent migration. Table 3 shows the results of univariate and multivariate analyses using the Cox proportional hazards model, using which the risk factors related to stent migration were investigated. The cutoff value for the length of bile duct stenosis was calculated using a receiver operating characteristic curve. Significant risk factors for stent migration in univariate analysis were the prior cancer treatment to procedure (hazard ratio [HR] 3.19; 95% confidence interval [CI], 1.10–13.50; p=0.03) and the length of bile duct stenosis <2.2 cm (HR, 3.00; 95% CI, 1.36–7.06; p=0.006). Multivariate analysis revealed that the length of bile duct stenosis <2.2 cm was the only significant risk factor for stent migration (HR, 2.58; 95% CI, 1.16–6.14; p=0.02).
When the analysis was limited to patients with a length of bile duct stenosis of 2.2 cm or more, RBO occurred in 39.5% (17/43) of cases, and the cause of RBO was distal migration in 14.0% (6/43) of cases, proximal migration in 9.3% (4/43), sludge obstruction in 7.0% (3/43), overgrowth in 2.3% (1/43), and others in 7.0% (3/43). Compared to the group with bile duct stenosis <2.2 cm, no significant differences were observed in stent length, prior drainage, prior cancer therapy, cancer treatment after the procedure, or cancer stage; in contrast, the migration rate was significantly lower (10/43 [23.3%] vs. 16/30 [53.3%], p=0.004) (Table 4). The median TRBO of the patients with a length of bile duct stenosis of 2.2 cm or more and others was 276 days (Fig. 3A) and 158 days (Fig. 3B), respectively. The non-obstruction rate at 6 months of patients with a length of bile duct stenosis of 2.2 cm or more and others was 68% (Fig. 3A) and 55% (Fig. 3B), respectively. Although not statistically significant, patients with bile duct stenosis of 2.2 cm or more tended to have longer TRBO than others (p=0.068).
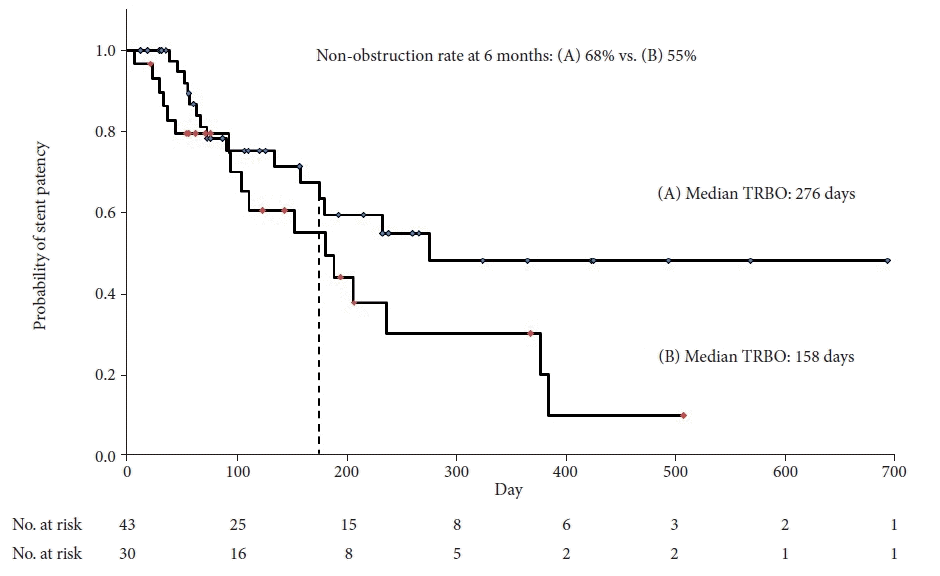
The Kaplan–Meier curve shows the time to recurrent biliary obstruction (TRBO) of the patients with a length of bile duct stenosis of 2.2 cm or more (A) and others (B). The median TRBO was 276 days (A) and 158 days (B). Although not statistically significant, patients with a length of bile duct stenosis of 2.2 cm or more tended to have a longer TRBO than others (p=0.068).
Reintervention
Reintervention was performed in 35 (47.9%) patients. The reasons for reintervention were RBO (n=33) and cholecystitis (n=2). Reintervention was successful in all cases. The procedure types for reintervention were uncovered SEMS placement (n=15), covered SEMS placement (n=11), plastic stent placement (n=7), and endoscopic sludge removal (n=2). SEMS removal was successful in 10 of 11 patients (90.9%). In one case of SEMS removal, duodenal perforation caused by the endoscope occurred but was successfully treated with endoscopic clipping. The median time to reintervention was 91 days (range, 6–385 days).
DISCUSSION
This study investigated the clinical performance of a novel, fully covered SEMS for treating unresectable MDBO. The results showed that the non-obstruction rate at 6 months was comparable to that reported earlier, but the results were not satisfactory.4,5,10-14 The high frequency of stent migration is thought to be the reason for inadequate stent patency. The length of bile duct stenosis <2.2 cm was the only significant risk factor for stent migration.
Recent progress in chemotherapy for unresectable malignant tumors is remarkable. Biliary drainage for MDBO is important not only for improving quality of life but also for prognostic purposes. Endoscopic biliary drainage is a widely accepted standard treatment for MDBO. The superiority of SEMSs over plastic stents for unresectable MDBO has been reported in many studies and their evaluation has already been established.2,15,16 Whether covered or uncovered SEMSs are superior remains unclear. The results of the present study are comparable with those of a recent systematic review.3 However, a recent study reported the results of fully covered SEMSs showing long TRBO with low migration rates. Yamao et al.17 reported a TRBO of 536 days, RBO of 14%, and migration of 4% for fully covered SEMS in the treatment of MDBO, whereas Marui et al.18 reported a TRBO of 445 days, RBO of 15%, and migration of 4%. With the recent emergence of covered SEMSs with longer TRBO, large-scale comparative studies are needed to clarify the superiority of novel covered SEMSs over uncovered SEMSs.
Stent migration is a common adverse event encountered in covered SEMSs.19 However, few studies have reported the risk factors for migration. Nakai et al.6 investigated risk factors for stent migration in patients with unresectable pancreatic cancer who underwent covered SEMS placement for MDBO. Covered SEMSs with a low RF, duodenal invasion, and chemotherapy have been reported as risk factors for stent migration. In the present study, stricture length (2 cm) was not found to be a significant risk factor for migration. However, stent migration is common in cases of ampullary cancer and is thought to be due to short stenosis.20 To our knowledge, our study is the first to report that bile duct stenosis of <2.2 cm is a risk factor for stent migration. Based on this finding, a fully covered SEMS should be avoided in patients with MDBO and short stenosis lengths.
The results of this study showed that the novel, fully covered SEMS migrated at a high frequency. This may have been due to the mechanical properties of the stents. Isayama et al.21 speculated that SEMS with high AF might not fit and stay well in the bile duct, thus causing migration. Although the RF and AF of this stent have not yet been investigated, a high AF may cause migration (Supplementary Fig. 1). Further investigation of the mechanical properties of these stents is required. However, the reported advantages of fully covered SEMSs are that ingrowth is less likely to occur and the stent can be easily removed by reintervention. The results of the current study are comparable with those of previous studies.
This study has several limitations. A major limitation is a single-arm design with a small sample size. Another limitation is that the clinical performance of this novel stent is comparable to that of pre-existing stents, but does not provide the expected clinical benefit. The strength of this study was the multicenter prospective design. Further, this is the first report to show that bile duct stenosis of <2.2 cm is a risk factor for stent migration.
In summary, the non-obstruction rate of a novel fully covered biliary SEMS for MDBO is comparable to that reported earlier but shorter than expected. The length of bile duct stenosis (<2.2 cm) was the only significant risk factor for stent migration.
Supplementary Material
Supplementary Fig. 1. A patient with stage 4 pancreatic cancer. A fully covered self-expandable metal stent was placed (A). One day later, an abdominal X-ray showed that the stent was almost fully dilated and was straightened to change the shape of the bile duct (B). Six months later, the stent was migrated distally. Another patient with stage 4 pancreatic cancer received a woven hook and cross-structured fully covered self-expandable metal stent (C). One day later, an abdominal radiograph showed slight dilation of the stent but less change in the shape of the bile duct (D).
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2023.035.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
This work was supported by JSPS KAKENHI (grant number JP19K07938 [AS]). The study sponsors did not play any role in the study design, collection, analysis, and interpretation of the data, or in writing the report.
Author Contributions
Conceptualization: AS, AM; Data curation: AS, TE, KF, TI, SY, YO, KY, IM, SKa, YY, DS, SKo; Formal analysis: AS, AM; Funding acquisition: AS, YK; Project administration: AS, AM, TK, HS; Supervision: YK; Writing‒original draft: AS; Writing‒review & editing: all authors.