Safety of endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction and ascites
Article information
Abstract
Background/Aims
Endoscopic ultrasound (EUS)-guided hepaticogastrostomy (EUS-HGS) is useful for patients with biliary cannulation failure or inaccessible papillae. However, it can lead to serious complications such as bile peritonitis in patients with ascites; therefore, development of a safe method to perform EUS-HGS is important. Herein, we evaluated the safety of EUS-HGS with continuous ascitic fluid drainage in patients with ascites.
Methods
Patients with moderate or severe ascites who underwent continuous ascites drainage, which was initiated before EUS-HGS and terminated after the procedure at our institution between April 2015 and December 2022, were included in the study. We evaluated the technical and clinical success rates, EUS-HGS-related complications, and feasibility of re-intervention.
Results
Ten patients underwent continuous ascites drainage, which was initiated before EUS-HGS and terminated after completion of the procedure. Median duration of ascites drainage before and after EUS-HGS was 2 and 4 days, respectively. Technical success with EUS-HGS was achieved in all 10 patients (100%). Clinical success with EUS-HGS was achieved in 9 of the 10 patients (90%). No endoscopic complications such as bile peritonitis were observed.
Conclusions
In patients with ascites, continuous ascites drainage, which is initiated before EUS-HGS and terminated after completion of the procedure, may prevent complications and allow safe performance of EUS-HGS.
INTRODUCTION
Endoscopic ultrasound-guided biliary drainage (EUS-BD) is a relatively new therapeutic modality that is used when conventional endoscopic retrograde cholangiopancreatography (ERCP) is not feasible.1-4 EUS-BD, which was first reported in 2001,5 has shown high success rates.6 EUS-BD is an alternative to percutaneous transhepatic biliary drainage (PTBD) and has been used to treat patients with biliary cannulation failure or inaccessible papillae. Its applicability in primary drainage has also been reported.7-9
EUS-BD comprises antegrade stenting, rendezvous with ERCP, and bilioenterostomy, and is categorized as EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS).10 Among these two techniques, EUS-HGS has the widest indications, including high-grade hilar stenosis, duodenal stenosis, surgically altered anatomy, and bile duct catheterization failure.11-14 Therefore, EUS-HGS is the most frequently performed EUS-BD procedure.15,16
Unlike ERCP, EUS-HGS avoids traumatic papillary irritation, which could lead to acute pancreatitis. Furthermore, unlike PTBD, it is more comfortable and physiologically safer for patients.
EUS-HGS-related complications, including bile leakage, peritonitis, stent prolapse, perforation, and bleeding, have been reported.17,18 In particular, the risk of severe complications is higher in patients with ascites than in those without ascites. Thus, to avoid complications, ERCP is preferentially performed instead of EUS-HGS in patients with ascites. However, ERCP may not be feasible in patients with stomach or duodenal invasion or in those with surgically altered anatomy. Thus, it is important to develop a safe method to perform EUS-HGS in patients with ascites. We hypothesized that EUS-HGS could be performed safely in patients with ascites by initiating continuous ascites drainage prior to the procedure and terminating it after completion of the procedure. Thus, herein, we evaluated whether EUS-HGS can be safely performed in patients who undergo continuous ascites drainage that was initiated before EUS-HGS and terminated after the completion of the procedure.
METHODS
Ten consecutive patients with moderate or severe ascites who had undergone EUS-HGS with continuous ascites drainage, which was initiated before EUS-HGS and terminated after its completion, between April 2015 and December 2022 at our institute were retrospectively investigated.
Patients with moderate or severe ascites who were likely to experience difficulty with PTBD, those with ascites in the EUS-HGS puncture route as observed on computed tomography (CT), and those who could safely undergo percutaneous ascites drainage underwent continuous ascites drainage. EUS-HGS was not originally indicated for patients with severe ascites; it was performed only when it was considered beneficial and the patient strongly desired advanced treatment.
CT of the abdomen and pelvis was performed 24 hours after the procedure to confirm the absence of complications such as stent migration and bile leakage. Follow-up began on the date of the first endoscopic drainage procedure and ended in December 2022 or on the date of the patient’s death.
Ascites drainage
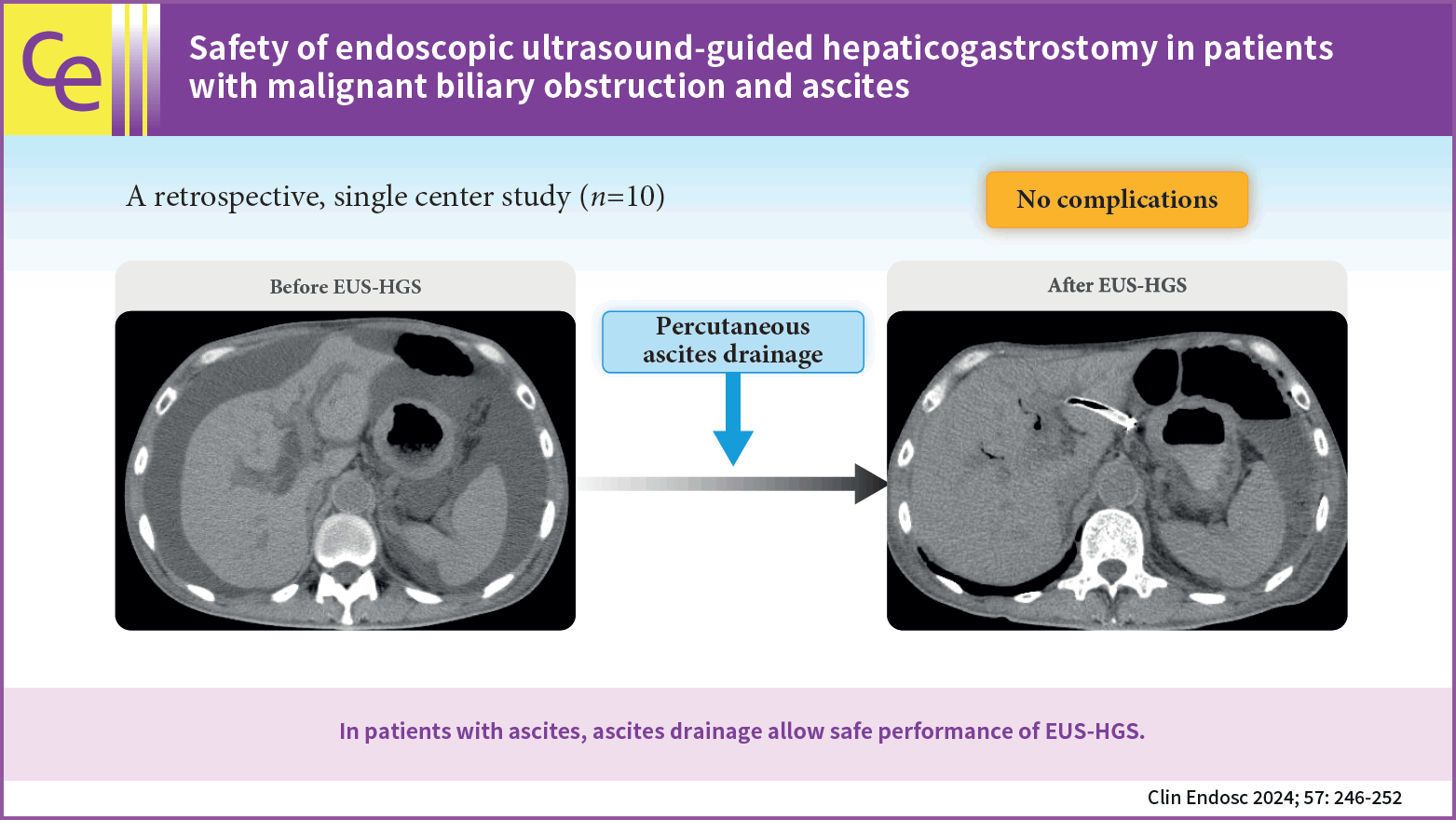
The daily upper limit of ascites drainage was 3,000 mL, and drainage was performed for up to 3 days before EUS-HGS. Drainage was stopped post-EUS-HGS when the volume had reached ≤1,000 mL/day and no infection was observed. The amount of ascites was evaluated using CT as per previously reported methods19,20 and was defined as follows: mild ascites, detected only in the upper or lower abdominal cavity; moderate ascites, detected in both the upper and lower abdominal cavities; and severe ascites, ascites extending continuously from the pelvic cavity to the upper abdominal cavity (Fig. 1).
Endoscopic procedures
All the procedures were performed under ultrasonographic and fluoroscopic guidance. EUS-HGS was performed using a convex echoendoscope (GF-UCT260; Olympus or EG-760UT, Fujifilm). Before EUS-HGS, a forward-viewing scope was used to clip the gastroesophageal junction for easy identification of the esophagus and to prevent its puncture. An echoendoscope was inserted into the stomach. After the puncture point was determined, the left intrahepatic bile duct (B2 or B3) was punctured using a 19 G needle (EZ shot 3 plus; Olympus) attached to a connector (Radifocus Hemostasis Valve II; Terumo). Subsequently, a 0.025-inch guidewire (M-through; Asahi Intecc) was introduced into the left intrahepatic bile duct. A small amount of contrast medium was injected to confirm appropriate placement of the guidewire. Thereafter, the guidewire was placed in the common bile duct and a fistula was created using a 7-Fr tapered cannula (ES dilator soft type, 0.025-inch; Zeon Medical). Cholangiography was performed using an uneven double-lumen cannula (Piolax Medical). Finally, a fully covered, self-expandable metal stent (6 mm×12 cm, Hanarostent; Boston Scientific) was placed and the implantation site was confirmed with fluoroscopy. In some cases, the devices were changed at the discretion of the surgeon.
Evaluated outcomes
The evaluated outcomes included the technical and clinical success rates of EUS-HGS, EUS-HGS-related complications, and feasibility of re-intervention.
Definitions
Technical success was defined as successful placement of the stent via the stomach into the left intrahepatic bile duct, which was confirmed by endoscopy and CT. Clinical success was defined as improvement in cholangitis or reduction in the total serum bilirubin level to <75% of the pretreatment value within 2 weeks. Procedure time was defined as the interval between the insertion of the scope and the completion of all procedures.
Complications
Complications of endoscopic treatment were divided into early (occurring within 14 days of the endoscopic procedure) and late (occurring >14 days after the procedure). These complications were evaluated according to the American Society for Gastrointestinal Endoscopy lexicon.21 Bleeding was defined as a reduction in the serum hemoglobin level to <2.0 g/dL from the pretreatment value.
Ethical statements
This study was approved by the Institutional Review Board of the Aichi Cancer Center Hospital (No. IR041150). The requirement for informed consent from patients was waived owing to the retrospective nature of this study.
RESULTS
The characteristics of the 10 patients are presented in Table 1. Of the 10 patients (six males, four females; median age, 66.5 [58–77] years) with malignant biliary obstruction, EUS-HGS had to be performed due to gastric or duodenal obstruction during the course of cancer in five (50%) patients, surgically altered anatomy in three (30%) patients, and lack of ERCP efficacy (bile duct catheterization failure despite some attempts) in two (20%) patients. The ascites was moderate in four (40%) patients and severe in six (60%) patients.

Clinical characteristics of patients who underwent continuous ascites drainage beginning before and ending after endoscopic ultrasound-guided hepaticogastrostomy
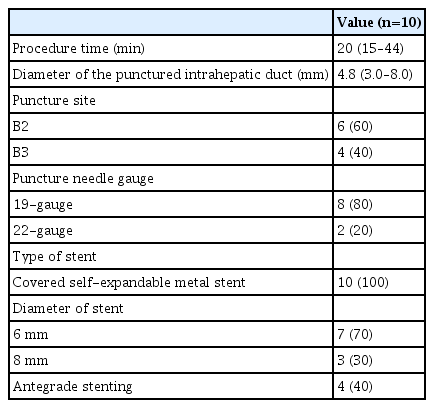
Details of the EUS-HGS procedure are shown in Table 2, and the clinical outcomes are shown in Table 3.
The median duration of pre- and post-EUS-HGS ascites drainage was 2 days (range, 1–3 days) and 4 days (range, 2–6 days), respectively. Antimicrobial agents were administered to all the patients after EUS-HGS. The median duration of post-EUS-HGS antimicrobial agent use was 7 days (range, 2–12 days), and the median duration until the resumption of eating was 1 day (range, 1–4 days).
Technical success was achieved in all 10 patients (100%). The median procedure time was 20 minutes (range, 15–44 mins). The median diameter of the punctured intrahepatic bile duct was 4.8 mm (range, 3.0–8.0 mm) and B2 and B3 punctures were performed in six and four patients, respectively. The gauge of the puncture needles used was 19-G in eight patients and 22-G in two patients. All stents were self-expandable metal stents.
Clinical success was achieved in nine of the 10 (90%) patients. EUS-HGS was clinically unsuccessful in one patient whose general condition worsened owing to the rapidly progressing advanced cancer. Chemotherapy was performed in patients in whom clinically successful endoscopic treatment was achieved because the total serum bilirubin level dropped below the threshold. This facilitated chemotherapy in two patients, whereas the other patients were administered the best supportive care because of their decreased performance status. The median follow-up duration after EUS-HGS was 43 days (range, 13–383 days), and the median stent patency was 43 days (range, 13–215 days).
No patients experienced endoscopic treatment-related early complications, such as bile peritonitis, infectious peritonitis, or stent migration (Supplementary Video 1, 2). During hospitalization, none of the patients had electrolyte abnormalities or renal dysfunction, and no late complications were noted (Table 4).
During long-term follow-up, three (30%) patients underwent re-intervention for cholangitis (n=2) or stent dislocation (n=1). The patients with cholangitis had complete fistulae; therefore, stent replacement was possible. Stent dislocation was observed on CT in one patient 203 days after the endoscopic procedure. Re-stent placement was challenging because of fistula closure; however, jaundice did not exacerbate after the procedure because chemotherapy relieved the bile duct stricture.
DISCUSSION
In patients with ascites who require biliary drainage, ERCP is preferred to EUS-HGS or PTBD because it does not typically cause bile leakage. However, this procedure may not be feasible if there is duodenal invasion or when the anatomy is surgically altered. In such cases, EUS-HGS can be used as an alternative. We hypothesized that EUS-HGS could be performed safely in patients with ascites if continuous ascites drainage is performed prior to, during, and after the procedure to minimize ascitic fluid volume.
To the best of our knowledge, no comprehensive studies on EUS-HGS have been performed in patients with ascites, and this is the first report to examine the safety of EUS-HGS in patients with ascites.
One limitation of the extrahepatic approach is the potentially high risk of bile leakage.22 In particular, patients with excess ascites have higher risks of developing peritonitis and stent migration because of the inability to form a mature fistula after the procedure.23 The overall EUS-HGS-related complication rate is approximately 18.2%, with bleeding (3.7%), bile leakage (2.8%), stent migration (2.8%), biloma (2.6%), liver hematoma (1.2%), and sepsis (1.2%) being more common.10 Bile leakage into the abdominal cavity and stent migration are the most serious complications of this procedure.15,24-26 Ascites carries the potential risk of complications, such as infectious peritonitis, which are occasionally serious. We previously reported a case of severe infectious peritonitis after EUS-HGS in a patient with ascites.27 Thus, it is important to prevent serious complications when performing EUS-HGS in patients with ascites.
Herein, initiating continuous ascites drainage before EUS-HGS and terminating it after the completion of the procedure resulted in technical and clinical success in 10 (100%) and 9 (90%) patients, respectively. No serious complications, such as bile peritonitis, were observed. Continued ascites drainage post-procedure until the volume was <1,000 mL/day may have also prevented the development of ascites between the stomach and liver and promoted fistula formation. Furthermore, preventing complications such as ascites infection and internal migration may have contributed as well. Thus, when EUS-HGS is scheduled for patients with ascites, percutaneous insertion of a drainage tube and continuous ascites drainage prior to, during, and after the procedure may prevent complications such as peritonitis and stent migration.
Bile leakage has been observed more frequently in patients receiving plastic stents (10%) than in those receiving covered, self-expandable, metal stents (4%)6; therefore, covered metal stents are recommended for patients with ascites. Luminal stent length is reportedly an important factor in reducing EUS-HGS-related adverse events.28 Therefore, considering stent shortening, a stent of ≥10 cm in length should be used to prevent stent migration.28,29 Herein, covered, self-expandable, metal stents (≥10 cm) were used in all patients. The use of migration-resistant stents may shorten the post-EUS-HGS ascites drainage period, and further increase the safety of the procedure.
Our study had some limitations. This single-center study included only a select group of nonrandomized patients. Additional studies are required to further evaluate the efficacy and safety of EUS-HGS with continuous ascites drainage prior to, during, and after the procedure in patients with malignant biliary obstruction and ascites.
In conclusion, EUS-HGS can be performed more safely in patients with malignant biliary obstruction and moderate or severe ascites by reducing the ascitic fluid volume via continuous drainage before and during EUS-HGS, which is terminated after the completion of the procedure.
Supplementary Material
Supplementary Video 1. Computed tomography imaging before endoscopic ultrasound-guided hepaticogastrostomy (http://doi.org/10.5946/ce.2023.075.v001).
Supplementary Video 2. Computed tomography imaging after endoscopic ultrasound-guided hepaticogastrostomy (http://doi.org/10.5946/ce.2023.075.v002).
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2023.075.
Notes
Conflicts of Interest
Dr. Mizuno reports receiving grants and personal fees from Yakult Honsha, Novartis, MSD, and ASLAN Pharmaceuticals, grants from Incyte, Ono Pharmaceutical, Seagen, Taiho Pharmaceutical, and Dainippon Sumitomo Pharma, and personal fees from AstraZeneca and Fujifilm Toyama Chemical for work other than the submitted paper. The authors declare no conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: TY, KH; Data curation: TY; Investigation: TY; Methodology: KH; Project administration: KH; Resources: TY; Supervision: KH; Validation: all authors; Visualization: TY; Writing–original draft: TY; Writing–review & editing: all authors.