AbstractBackground/Aims:Scissor-type endoscopic submucosal dissection (ST-ESD) knives can reduce the adverse events associated with ESDs. This study aimed to compare ST-ESD and non-scissor-type (NST)-ESD knives.
Methods:We identified ten studies that compared the performance characteristics and safety profiles of ST-ESD and NST-ESD knives. Fixed- and random-effects models were used to calculate the pooled proportions. Heterogeneity was assessed using the I2 test.
Results:On comparing ST-ESD knives to NST-ESD knives, the weighted odds of en bloc resection was 1.61 (95% confidence interval [CI], 0.90ŌĆō2.90; p=0.14), R0 resection was 1.10 (95% CI, 0.71ŌĆō1.71; p=0.73), delayed bleeding was 0.40 (95% CI, 0.17ŌĆō0.90; p=0.03), perforation was 0.35 (95% CI, 0.18ŌĆō0.70; p<0.01) and ESD self-completion by non-experts was 1.89 (95% CI, 1.20ŌĆō2.95; p<0.01). There was no heterogeneity, with an I2 score of 0% (95% CI, 0%ŌĆō54.40%).
Conclusions:The findings of reduced odds of perforation, a trend toward reduced delayed bleeding, and an improvement in the rates of en bloc and R0 resection with ST-ESD knives compared to NST-ESD knives support the use of ST-ESD knives when non-experts perform ESDs or as an adjunct tool for challenging ESD procedures.
INTRODUCTIONEndoscopic submucosal dissection (ESD) for early cancers and large gastrointestinal luminal lesions is well-established in European and Asian countries.1,2 ESD is currently indicated for most superficial esophageal squamous cell tumors, Barrett's esophagus-associated lesions, and superficial gastric lesions.2 ESD is also recommended for en bloc resection of colorectal lesions with features suggestive of limited submucosal invasion (demarcated, depressed areas with irregular surface patterns, or large protruding or bulky components, particularly if the lesions are larger than 20 mm) or for lesions that otherwise cannot be entirely removed by snare-based techniques.2 ESD has also been used for the resection of duodenal and small bowel lesions, but is recommended only for selected cases in expert centers.2 ESD enables the endoscopist to achieve en bloc resection regardless of lesion size with complete (R0) resection, thus allowing for comprehensive pathological staging of the tumor.3,4 In contrast to Asian countries and Europe, ESD in the United States of America (USA) is primarily confined to major academic high-volume centers, where it is performed by therapeutic endoscopists, and the adoption of ESD has been gradual for various reasons.4,5 These include a steep learning curve, longer procedure time, limited training opportunities, and an increased risk of severe complications, such as bleeding and perforation, compared with snare-based resection techniques. With the establishment of its efficacy over the past two decades, increased training exposure, and technological advancements, ESD is gaining acceptance among gastroenterologists in the West, particularly in the USA.6
However, ESD is a technically challenging endoscopic procedure with a risk of severe adverse events.7 ESD is a multistep process, involving various tools, and consists of the following steps: (1) the boundary of the lesion is marked using an electrosurgical knife; (2) a lifting solution is injected into the submucosal plane, raising the lesion; (3) a circumferential or semi-circumferential mucosal incision is performed around the lesion; and (4) the lesion is meticulously dissected along the submucosal plane using electrosurgical knives, separating it from the underlying muscle layer, and resected.6 Intraprocedural bleeding is common when performing ESDs and is usually managed in real-time using electrocoagulation. This may be performed with an electrosurgical knife using a soft coagulation setting or may necessitate the use of other tools, such as a hemostatic grasper. Hence, dissection and hemostatic devices play crucial roles in achieving positive ESD outcomes. Several electrosurgical knives are available today, most of which are needle-type (IT knife, hook knife, triangle tip knife, dual knife, flex knife, hybrid knife, etc.).7 Precise endoscopic tip control to keep pace with the dissection rate is critical to avoid unintended cutting, which can lead to adverse events such as perforation or bleeding. This can prove challenging depending on natural movements (peristalsis, respiration, heartbeat), approach angle, and the endoscopistŌĆÖs skill.8-10 Moreover, ESD has evolved from a procedure that was initially limited to gastric lesions to other, more challenging, and complex areas of the gastrointestinal tract, such as the duodenum and right colon. In 2007, a new scissor-type ESD (ST-ESD) knife was developed in Japan to overcome the limitations of needle-type knives.8,9 ST-ESD knives are unique in their ability to grasp and pull the target tissue away from the deeper muscle layer before electrosurgical cutting or coagulation.8 ST-ESD knives also have an external electrically insulated coating with sharply jagged, non-insulated inner cutting jaws.8,9 This allows the precise delivery of electrosurgical current to the grasped tissue while sparing adjacent regions. Endoscopists can also use this grasping feature of the ST-ESD knife to control bleeding as it can perform the functions of hemostatic forceps using a soft coagulation setting.9 These qualities of the ST-ESD knife have been shown to reduce adverse events such as severe postprocedural bleeding and perforations, specifically when non-experts perform ESDs. The Stag-beetle (SB) knife shown in, Figure 1 (Sumitomo Bakelite) and the ClutchCutter (CC) knife shown in Figure 2 (Fujifilm Medical) are the two types of ST-ESD knives currently available.8,9 Studies have compared the efficacy and safety of ST-ESD knives with non-scissor-type ESD (NST-ESD) knives with conflicting findings. For example, in an earlier study, Akahoshi et al.9 reported that using ST-ESD knives was time-consuming compared to NST-ESD knives. However, studies by Hayashi et al.,11 Dohi et al.,12 Esaki et al.,13 and Inoue et al.14 found that the use of an ST-ESD knife was associated with a significantly lower total procedure time when performed by less experienced endoscopists. Studies by Oka et al.,10 Fujinami et al.,15 and Kuwai et al.16 found significant improvements in en bloc and R0 resection rates with a reduction in perforation and bleeding rates, while other studies noted no statistically significant difference in these parameters.11-14,17-19
METHODSSearch methodologyA literature search was performed using the electronic databases Medline through PubMed, Ovid, Cochrane Library (Cochrane Central Register of Controlled Trials and Cochrane Database of Meta-Analysis), Embase, and the Database of Abstracts of Reviews of Effects according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines from January 2005 to October 2022 to identify studies comparing the performance of ST-ESD knives to NST-ESD knives. The keywords used were ŌĆ£endoscopic submucosal dissectionŌĆØ, ŌĆ£ESDŌĆØ, ŌĆ£ESD knifeŌĆØ, ŌĆ£grasping-type ESD knifeŌĆØ, "scis┬Łsor-type ESD knifeŌĆØ, ŌĆ£ClutchCutterŌĆØ, ŌĆ£SB knifeŌĆØ, and ŌĆ£insulated tip knifeŌĆØ. The references of the reviewed articles were scanned for additional studies. The retrieved studies were carefully examined to exclude potential duplicates or overlapping data.
Study eligibilityPublished studies were eligible if they compared scissor-type knives to conventional needle- or blade-type electrosurgical knives for ESDs. Articles were excluded if they were not published in English. Studies on animal models, editorials, abstracts with incomplete data, and comments were excluded. Ten articles matched the study criteria, and two authors independently reviewed the full-text articles (HG and IV). Differences were resolved by mutual agreement or review by a third author (SRP). Interobserver agreement was evaluated using CohenŌĆÖs ╬║.
Data extraction and quality assessmentThe following data were independently abstracted by two authors (HG and IV) into a standardized form: study characteristics (primary author, period of study, year of publication, and country of the population studied), study design, baseline characteristics of the study population (number of patients enrolled and participant demographics), intervention details (number of ESD procedures, location, indication), outcomes (en bloc resection, R0 resection, duration of procedures, and speed of procedure), adverse events (perforations and delayed bleeding), and operator experience.
Outcomes evaluatedThe primary outcomes evaluated were en bloc resection rates, R0 resection rates, and total procedure time. The adverse events compared were the perforation and delayed bleeding rates.
Statistical analysisThis meta-analysis was performed by calculating the weighted pooled effects. Individual study proportions were transformed into quantities using the Freeman-Turkey variant of the arcsine square root-transformed proportion. The pooled proportion was calculated as the back-transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed-effects model (Mantel-Haenszel method) and the random-effects model (DerSimonian-Laird method). The heterogeneity of the studies was evaluated using CochranŌĆÖs Q test based on inverse variance weights and by calculating I2 statistics. I2 values of 0% to 39% were considered non-significant heterogeneity, 40% to 75% moderate heterogeneity, and 76% to 100% considerable heterogeneity. A p-value >0.10 was used to reject the null hypothesis that the studies were heterogeneous. The findings of this meta-analysis were reported using a fixed-effects model as there was no statistically significant heterogeneity. Forest plots were drawn to show the point estimates for each study relative to a summary of the pooled estimates. The width of the point estimates in the forest plots indicates the weight assigned to that study. The odds ratio was used to represent dichotomous outcomes with a 95% confidence interval (CI), where a p-value of <0.05 was considered statistically significant. The effects of publication and selection biases on the summary estimates were tested using the Egger and Begg-Mazumdar bias indicators. Funnel plots were constructed to assess the potential publication bias using standard errors and diagnostic odds ratios. The quality of the included studies was evaluated using the Newcastle-Ottawa scale for nonrandomized studies and the Jadad scale for randomized controlled trials. Microsoft Excel 2019 was used to perform the statistical analysis.
RESULTSThe initial search identified 1,793 studies, of which 85 were relevant. Data were extracted and analyzed from ten studies that met the inclusion criteria. These studies included 1,831 patients who underwent 1,960 ESD procedures. In studies that used propensity-matched analysis, data reported from the propensity-matched cohorts were used for the final analysis. The PRISMA outline describing the details of the review process are shown in Figure 3. All the included studies are available as full-text articles. The studies included in this meta-analysis and their characteristics are summarized in Table 1.10-19 The quality of the studies was good, as evaluated using the Newcastle-Ottawa and Jadad scales shown in Tables 2, 3,10-19 respectively. All pooled estimates were calculated using a fixed-effects model, because there was no significant heterogeneity. The agreement between reviewers was 1.0, as measured by CohenŌĆÖs ╬║.
The total sample size was 1,960 ESD procedures. ST-ESD knives were used in 768 ESD procedures, whereas NST-ESD knives alone were used in the other 1,192 ESDs. The mean patient age was 70.01┬▒1.73 years in the ST-ESD group and 69.78┬▒1.65 years in the NST-ESD group. The overall pooled lesion size in the ST-ESD group was 28.46┬▒7.93 mm and 29.92┬▒6.20 mm in the NST-ESD group. Pooled lesion size for colorectal lesions was 31.84┬▒4.94 mm in the ST-ESD group and 32.00┬▒2.96 mm in the NST-ESD group. Table 4 summarizes the findings. CC knife was the ST-ESD knife used in five studies, SB knife (SB and SB knife Jr [SB Jr], MD-47703; Sumitomo Bakelite) was used in four studies, and Kuwai et al.16 used both the ClutchCutter and SB knife. Three of the ten studies were prospective randomized controlled trials, whereas the others were retrospective studies.
Analysis showed that the weighted odds of en bloc resection with an ST-ESD knife compared to an NST-ESD knife was 1.61 (95% CI, 0.90ŌĆō2.90; p=0.14), while the weighted odds of an R0 resection with an ST-ESD knife compared to an NST-ESD knife was 1.10 (95% CI, 0.71ŌĆō1.71; p=0.73). There was no heterogeneity, with an I2 scores of 0% (95% CI, 0%ŌĆō56.30%) and 0% (95% CI, 0%ŌĆō58.50%) for en bloc and R0 resections, respectively. Figure 4 shows a forest plot comparing the odds of en bloc resection, and Figure 5 shows the odds of R0 resection between ST-ESD and NST-ESD knives.10,12-19 The weighted odds of en bloc and R0 resections based on the anatomic location of the ESD were also analyzed. This showed that the weighted odds of en bloc resection for colorectal lesions with an ST-ESD knife compared to an NST-ESD knife was 1.95 (95% CI, 0.99ŌĆō3.81; p=0.06), and for gastric lesions was 0.21 (95% CI, 0.02ŌĆō2.00; p=0.31). Analysis of adverse events showed that the weighted odds of delayed bleeding with an ST-ESD knife compared to an NST-ESD knife was 0.40 (95% CI, 0.17ŌĆō0.90; p=0.03), while the weighted odds of delayed perforation was 0.35 (95% CI, 0.18ŌĆō0.70; p<0.01). The forest plot showing the individual study estimates and the pooled estimate for delayed perforation is shown in Figure 6.10,12-19 There was no heterogeneity, with an I2 score of 0% (95% CI, 0%ŌĆō54.40%). The Begg-Mazumdar bias indicator yielded a Kendall's tau b value of 0 (p=0.91), suggesting no publication bias. Figure 7 shows the funnel plot assessing publication bias for the odds of perforation when comparing ST-ESD to NST-ESD knives. The weighted odds of self-completion of ESD by non-experts was 1.89 (95% CI, 1.20ŌĆō2.95; p<0.01) when using an ST-ESD knife compared to an NST-ESD knife. A forest plot showing the odds of self-completion is shown in Figure 8. The pooled overall ESD procedure completion time was 63.03 minutes (95% CI, 60.77ŌĆō65.29) in the ST-ESD knife group and 65.19 minutes (95% CI, 63.31ŌĆō67.06) in the NST-ESD knife group. On evaluation based on location, the procedure completion time was 64.89 minutes (95% CI, 62.15ŌĆō67.63) in the ST-ESD knife group compared to 65.95 minutes (95% CI, 63.86ŌĆō68.05) in the NST-ESD group for colorectal ESDs.
DISCUSSIONThe main finding of this meta-analysis was that the performance of ST-ESD knives was comparable to that of conventional NST-ESD knives, with similar rates of en bloc and R0 resections. However, there was a statistically significant reduction in the odds of perforation with the ST-ESD knife compared with the NST-ESD knife. A trend favoring the ST-ESD knife for mitigating delayed bleeding was also observed.
Increased risk for significant complications, including perforation and bleeding, has been a major deterrent to ESD adoption in the USA, as discussed by Schlachterman et al.5 in their survey on ESD training in the USA. Previous studies using NST-ESD knives have reported varying perforation rates of 4.3% to 8.2% following gastric ESDs,20,21 approximately 4% following esophageal ESDs,22 and approximately 5% following colonic ESDs.23-25 Duodenal ESDs carry the highest risk of perforation, with a wide variability in reported rates.26 Our analysis showed that the odds of perforation were significantly lower with ST-ESD knives than with NST-ESD knives. Electrosurgical incision using a conventional needle-type knife is achieved by the controlled lateral movement of the cutting edge of the needle, which requires a high level of precision and skill from the endoscopist. This mechanism differs from that of familiar endoscopic tools, which primarily involve open and closed tip motions (e.g., biopsy forceps, clips, and snares).27 Scissor-type ESD knives work like other familiar endoscopic tools using a similar grasp-and-cut approach. The target tissue is also pulled away from the underlying tissue before electrosurgical dissection. Both these mechanisms, in addition to the electrically insulated outer coating of ST-ESD knives, mitigate the risk of injury to the deeper muscle layer.8,9,27 These factors could explain the lower perforation rates observed with ST-ESD knives, particularly in the hands of less experienced endoscopists. The odds of delayed bleeding were similar between ST-ESD and NST-ESD knives but showed a trend that favored ST-ESD knives. Owing to its ability to grasp tissue, an ST-ESD knife can also be used as coagulation forceps with soft coagulation settings. This could reduce the need for frequent exchange of tools when a vessel is encountered during the submucosal dissection step and reduce the overall risk of bleeding. None of the studies reported any adverse events that could be attributed to using an ST-ESD knife, aside from the standard adverse events seen in any ESD, regardless of the type of electrosurgical knife used. In addition to the type of endoscopic tool and operator inexperience, the risk of perforation and delayed bleeding also depends on factors such as tumor size, location, fibrosis, and procedure duration.21 Hence, an ST-ESD knife could be the preferred electrosurgical knife for lesions in high-risk locations, or could be used as an adjunct to conventional NST-ESD knives for challenging ESDs, as reported by Yamashina et al.18
The main advantage of ESD over snare-based resection techniques is the higher rate of en bloc and R0 resections, particularly for lesions >20 mm.20 Our study showed that the odds of en bloc and R0 resections were comparable between the ST-ESD and NST-ESD knives, with a trend observed to favor the ST-ESD knife, although the difference was not statistically significant. Our analysis also showed that the odds of achieving an en bloc resection for colorectal lesions with an ST-ESD knife compared to an NST-ESD knife was 1.95, while that for gastric lesions was 0.21. Colorectal ESD is considered more difficult than gastric ESD because the colorectal wall is thinner than that of the stomach.28 Depending on the location of the lesion, there can be additional challenges in performing colorectal ESD, such as the maneuverability of the endoscope in the colon (especially the right colon), physiological flexion, peristalsis, and respiratory movements.28 The improved odds of en bloc resection of colorectal lesions with ST-ESD knives may be due to these reasons, although the difference may not be prominent for gastric lesions. One criticism of the ST-ESD knife was that its use could increase the total procedure time. However, this meta-analysis of the pooled data did not show any significant difference in the total procedure time between ST-ESD and NST-ESD knives. The ST-ESD knife was used for all steps involving an electrosurgical knife in four of the included studies,13,14,16,17 and only one study reported using a NST knife to perform the mucosal incision step.19 This shows that an ST-ESD knife can be used as a standalone ESD knife or as an adjunct.
The findings of this study also suggest that the traineesŌĆÖ odds of self-completion of an ESD procedure are higher with ST-ESD knives than with non-ST-ESD knives. Dohi et al. found no significant difference in R0 resection between ST-ESD and NST-ESD knives in gastric ESDs when experts performed the procedure. However, there was a significant improvement in both self-completion rates and mean procedure time when non-experts performed ESD using ST-ESD knives.12 Yamashina et al.18 also observed an improvement in the self-completion rate when trainees performed ESDs using scissor-type knives. Head-to-head comparisons of these two types of ESD knives in trainees are scarce, because most available studies are based on expert experience. Further studies are required to confirm these findings. This has significant implications, particularly for ESD training programmes.
Accumulating evidence over the past two decades has proven that ESD is effective in achieving high en bloc and curative resection of lesions that are not amenable to conventional snare-based endoscopic resection techniques.20 Many factors, including a perceived higher rate of complications than EMR, a steep learning curve, and the slow release of ESD tools, have hampered the widespread adoption of ESD in the USA. ESDs have conventionally been performed using needle-type electrosurgical knives. The ST-ESD knife has been used in Japan over the past decade, and studies have evaluated its unique features and usefulness. This meta-analysis of studies comparing these two types of ESD knives further demonstrates that the performance of ST-ESD knives is comparable to that of conventional NST-ESD knives, with the potential to mitigate some of the complications associated with ESD. With the recent enthusiasm and increasing accessibility to ESD training, the findings of this study could help guide appropriate tool selection for ESD, particularly for beginners.
To the best of our knowledge, this is the first comprehensive meta-analysis comparing scissor-type and non-scissor-type knives for ESDs across various locations in the gastrointestinal tract. The findings of this study provide a compelling argument for including ST-ESD knives in an endoscopistŌĆÖs arsenal. Further prospective studies are needed to clarify its role, either as an initiation tool for trainees or as an adjunct based on the complexity of the procedure.
This study has a few limitations. All of these studies were performed in Japan, where the ESD technique was pioneered and has been in practice for over two decades. It is important to note that even non-experts in some of these studies were endoscopists with significant ESD experience. For example, in a study by Hayashi et al.,11 an endoscopist with up to 50 previous ESD procedures was still considered a non-expert. This is very different from the Western setting, particularly an ESD training program. Some of the findings of this study, such as procedure time, which can be significantly affected by expertise, should be interpreted with consideration of this fact. There were also insufficient data regarding specific parameters such as the depth of invasion of lesions and details on patient comorbidities, which limited our ability to evaluate these. Needle-type and other ESD knives have been in practice for a much longer time than scissor-type knives, and experts have experience with them. It is essential to recognize that a potential limitation of this study is that only a trend favoring ST-ESD knife in en bloc and R0 resections was seen. No superiority was demonstrated for this important primary endpoint. Further prospective studies comparing outcomes, primarily when trainees perform ESDs in Western endoscopy training programs, are warranted to evaluate the relevance of our findings.
CONCLUSIONSThe results of this meta-analysis showed a significant reduction in the odds of perforation with an ST-ESD knife compared to an NST-ESD knife. There was also a trend favoring the ST-ESD knife in reducing the risk of delayed bleeding and improving en bloc and R0 resection rates, although this was not statistically significant. These findings support the consideration of the ST-ESD knife as an additional tool to increase the safety of ESDs, particularly when performed by non-experts. It can also be a useful secondary tool for challenging ESD procedures.
NOTESFig.┬Ā3.Study flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. ST-ESD, scissor-type endoscopic submucosal dissection; NST-ESD, non-scissor-type endoscopic submucosal dissection. 
Fig.┬Ā4.Forest plot comparing the odds of en bloc resection between the ST-ESD and NST-ESD knives. ST-ESD, scissor-type endoscopic submucosal dissection; NST-ESD, non-scissor-type endoscopic submucosal dissection. 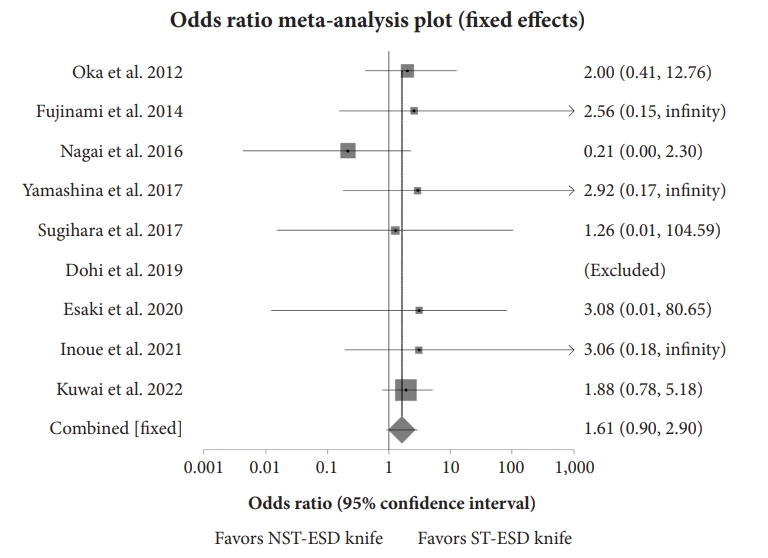
Fig.┬Ā5.Forest plot comparing the odds of R0 resection between the ST-ESD and NST-ESD knives. ST-ESD, scissor-type endoscopic submucosal dissection; NST-ESD, non-scissor-type endoscopic submucosal dissection. 
Fig.┬Ā6.Forest plot comparing the odds of delayed perforation between the ST-ESD and NST-ESD knives. ST-ESD, scissor-type endoscopic submucosal dissection; NST-ESD, non-scissor-type endoscopic submucosal dissection. 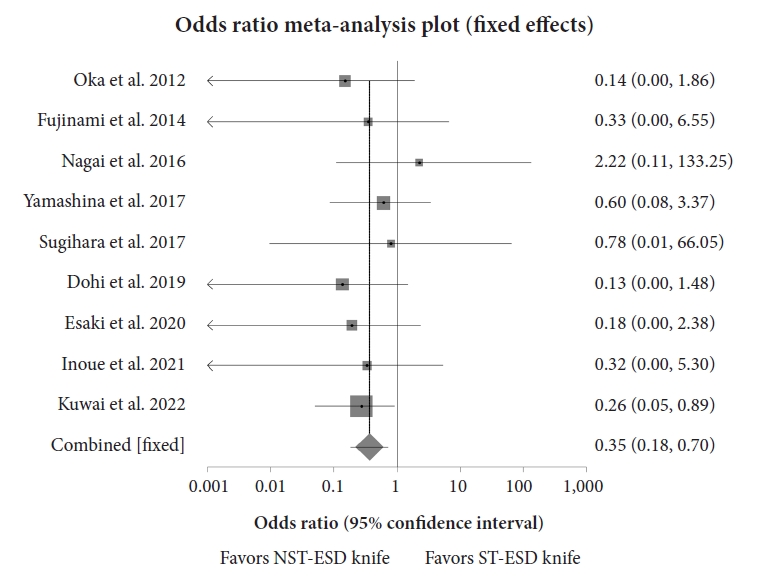
Fig.┬Ā8.Forest plot comparing the odds of self-completion of ESD when performed by non-experts using ST-ESD vs. NST-ESD knives. ST-ESD, scissor-type endoscopic submucosal dissection; NST-ESD, non-scissor-type endoscopic submucosal dissection. 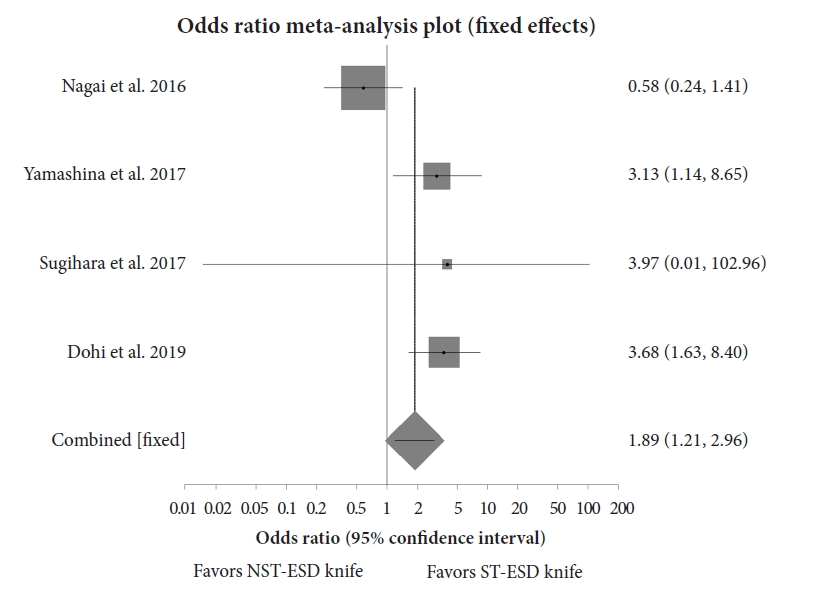
Table┬Ā1.Basic characteristics of studies included in this meta-analysis
ST-ESD, scissor-type endoscopic submucosal dissection; SB, Stag-beetle knife (Sumitomo Bakelite); SB Jr, Stag-beetle knife Jr (Sumitomo Bakelite); CC, ClutchCutter (Fujifilm Medical); RCT, randomized controlled trial. Table┬Ā2.Modified Newcastle-Ottawa scale assessing the quality of included non-randomized studies
Table┬Ā3.Jadad quality assessment tool for randomized control trials included in this meta-analysis
REFERENCES1. Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc 2021;33:4ŌĆō20.
2. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829ŌĆō854.
3. Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16ŌĆō25.
4. Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic submucosal dissection: indications and application in Western endoscopy practice. Gastroenterology 2018;154:1887ŌĆō1900.
5. Schlachterman A, Yang D, Goddard A, et al. Perspectives on endoscopic submucosal dissection training in the United States: a survey analysis. Endosc Int Open 2018;6:E399ŌĆōE409.
6. Yang D, Hasan MK, Draganov PV. East versus West: comparisons and implications in adaptation to practice. Gastrointest Endosc Clin N Am 2023;33:7ŌĆō13.
7. Ahmed Y, Othman M. EMR/ESD: techniques, complications, and evidence. Curr Gastroenterol Rep 2020;22:39.
8. Honma K, Kobayashi M, Watanabe H, et al. Endoscopic submucosal dissection for colorectal neoplasia. Dig Endosc 2010;22:307ŌĆō311.
9. Akahoshi K, Okamoto R, Akahane H, et al. Endoscopic submucosal dissection of early colorectal tumors using a grasping-type scissors forceps: a preliminary clinical study. Endoscopy 2010;42:419ŌĆō422.
10. Oka S, Tanaka S, Takata S, et al. Usefulness and safety of SB knife Jr in endoscopic submucosal dissection for colorectal tumors. Dig Endosc 2012;24 Suppl 1:90ŌĆō95.
11. Hayashi Y, Esaki M, Suzuki S, et al. Clutch Cutter knife efficacy in endoscopic submucosal dissection for early gastric neoplasms. World J Gastrointest Oncol 2018;10:487ŌĆō495.
12. Dohi O, Yoshida N, Terasaki K, et al. Efficacy of clutch cutter for standardizing endoscopic submucosal dissection for early gastric cancer: a propensity score-matched analysis. Digestion 2019;100:201ŌĆō209.
13. Esaki M, Hayashi Y, Ikehara H, et al. The effect of scissor-type versus non-scissor-type knives on the technical outcomes in endoscopic submucosal dissection for superficial esophageal cancer: a multi-center retrospective study. Dis Esophagus 2020;33:doz077.
14. Inoue K, Yoshida N, Dohi O, et al. Effects of the combined use of a scissor-type knife and traction clip on endoscopic submucosal dissection of colorectal tumors: a propensity score-matched analysis. Endosc Int Open 2021;9:E1617ŌĆōE1626.
15. Fujinami H, Hosokawa A, Ogawa K, et al. Endoscopic submucosal dissection for superficial esophageal neoplasms using the stag beetle knife. Dis Esophagus 2014;27:50ŌĆō54.
16. Kuwai T, Oka S, Kamigaichi Y, et al. Efficacy and safety comparison of scissor-type knives with needle-type knives for colorectal endoscopic submucosal dissection: a post-hoc propensity score-matched analysis (with videos). Gastrointest Endosc 2022;96:108ŌĆō117.
17. Nagai K, Uedo N, Yamashina T, et al. A comparative study of grasping-type scissors forceps and insulated-tip knife for endoscopic submucosal dissection of early gastric cancer: a randomized controlled trial. Endosc Int Open 2016;4:E654ŌĆōE660.
18. Yamashina T, Takeuchi Y, Nagai K, et al. Scissor-type knife significantly improves self-completion rate of colorectal endoscopic submucosal dissection: single-center prospective randomized trial. Dig Endosc 2017;29:322ŌĆō329.
19. Sugihara Y, Harada K, Kawahara Y, et al. Two electrosurgical endo-knives for endoscopic submucosal dissection of colorectal superficial neoplasms: a prospective randomized study. Endosc Int Open 2017;5:E729ŌĆōE735.
20. Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763ŌĆō770.
21. Watari J, Tomita T, Toyoshima F, et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: a prospective pilot study. World J Gastrointest Endosc 2013;5:281ŌĆō287.
22. Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 2012;75:1153ŌĆō1158.
23. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217ŌĆō1225.
24. Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014;79:427ŌĆō435.
25. Lee EJ, Lee JB, Lee SH, et al. Endoscopic submucosal dissection for colorectal tumors: 1,000 colorectal ESD cases: one specialized instituteŌĆÖs experiences. Surg Endosc 2013;27:31ŌĆō39.
26. P├®rez-Cuadrado-Robles E, Qu├®n├®herv├® L, Margos W, et al. ESD versus EMR in non-ampullary superficial duodenal tumors: a systematic review and meta-analysis. Endosc Int Open 2018;6:E998ŌĆōE1007.
|
|
