AbstractBackground/AimsRadiofrequency ablation (RFA) is the first-line therapy for dysplastic Barrettãs esophagus (BE). Therefore, cryotherapy has emerged as an alternative treatment option. This study aimed to compare the efficacies of these two techniques based on the rates of complete eradication of intestinal metaplasia (CE-IM) and dysplasia (CE-D). Adverse events and recurrence have also been reported.
MethodsAn electronic search was conducted using the Medline (PubMed), Embase, LILACS, and Google Scholar databases until December 2022. Studies were included comparing cryotherapy and RFA for treating dysplastic BE with or without early esophageal neoplasia. This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
ResultsThree retrospective cohort studies involving 627 patients were included. Of these, 399 patients underwent RFA, and 228 were treated with cryotherapy. There was no difference in CE-IM (risk difference [RD], ã0.03; 95% confidence interval [CI], ã0.25 to 0.19; p=0.78; I2=86%) as well as in CE-D (RD, ã0.03; 95% CI, ã0.15 to 0.09; p=0.64; I2=70%) between the groups. The absolute number of adverse events was low, and there was no difference in the recurrence rate.
INTRODUCTIONBarrettãs esophagus (BE) is characterized by a change from a normal squamous epithelium to a columnar epithelium with intestinal metaplasia.1 It is considered a premalignant condition owing to the established risk of developing dysplasia.2 Mucosa with dysplasia may progress to adenocarcinoma, a disease with an increasing incidence and low survival rates.3,4 Endoscopic eradication therapy (EET) of dysplastic areas prevents disease progression.5,6
Radiofrequency ablation (RFA) is considered first-line therapy.7 Previous studies demonstrated its high efficacy and safety. The rate of complete eradication of intestinal metaplasia (CE-IM) varied between 78% and 88%, while the complete eradication of dysplasia (CE-D) varied between 91% and 96%.8,9 More recently, the final 10-year UK National HALO Radiofrequency Ablation Registry report published in 2022 revealed a CE-IM of 62.7% and a CE-D of 88% at 2 years.10 Adverse events reported after RFA include stenosis, bleeding, and chest discomfort, although these are not very frequent.9-11
Cryotherapy has emerged as a treatment option in the last decade. There are three systems: liquid nitrogen, carbon dioxide, and a cryoballoon focal ablation system (nitrous oxide).12 Studies have revealed that CE-IM ranges from 64% to 82% and CE-D ranges from 82% to 94% when this method is primarily used.12,13 However, cryotherapy is usually considered a rescue alternative for patients who fail therapy.14,15 Cryotherapy is associated with less postprocedural pain than RFA, which may help with treatment adherence.16 Postprocedural stenosis has been described in some studies as an uncommon complication of cryotherapy.17
This meta-analysis was based on the clinical requirements of RFA and cryotherapy for treating dysplastic BE. This study compared the efficacy of RFA and cryotherapy using CE-IM and CE-D in adult patients with BE and histological evidence of low-grade dysplasia (LGD), high-grade dysplasia (HGD), or intramucosal adenocarcinoma (IMC). The number of adverse events and recurrences reported in these studies have also been described.
METHODOLOGYStudy protocol and registrationThis meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses checklist and registered in the International Prospective Register of Systematic Reviews PROSPERO (CRD42022374012).
Search strategyAn electronic search was conducted of the Medline (PubMed), Embase, LILACS, and Google Scholar databases, as well as a manual search of the references of the most relevant studies.
The search strategies were based on a combination of the term Barrett with descriptors referring to radiofrequency ablation (Catheter Ablation OR Radiofrequency OR Radio-Frequency OR Radio Frequency OR RFA) and Barrett with descriptors referring to cryotherapy (Cryotherapy OR Cold Therapy OR Cold Therapies OR Cryogen OR Cryosurgeries OR Cryoablation OR Cryoablations).
These were conducted between March 2022 and December 2022. There were no restrictions on the language or publication period, and full texts or abstracts were included. Alerts were created in these databases to obtain new results.
Selection criteriaThe following inclusion criteria were established (PICOS): (1) Population: adults with BE and histological evidence of LGD, HGD, or IMC; (2) Type of intervention/comparison: RFA versus cryotherapy; (3) Outcomes: CE-IM, CE-D, adverse events, and recurrence; (4) Study design: observational cohort or randomized clinical trials.
The following exclusion criteria were established (1) Determining the BE eradication rate by non-histopathological methods; (2) Absence of at least one surveillance endoscopy after completion of treatment.
Selection of studies and data extractionDuplicate studies were excluded from the analysis. Two independent reviewers read the titles and abstracts and discarded those that did not answer the research questions of interest. Full texts were read and selected based on inclusion and exclusion criteria. The reviewers reached a consensus during disagreements at any selection stage.
Data were independently extracted by two reviewers and recorded using specific collection forms. Information on study design, year, center, recruitment period, number of patients, demographic profile, mean maximum BE length, histology before ablation, CE-IM, CE-D, number of adverse events, and recurrence was collected. Divergence was resolved by consensus among the reviewers after retrieving information from the original article.
Outcomes assessedThe primary outcomes analyzed were the rate of CE-IM and the rate of CE-D. CE-IM is characterized by the absence of intestinal metaplasia on histopathological analysis of biopsies performed after treatment. Similarly, CE-D is defined by the absence of dysplasia from the histopathological analysis of biopsies taken after treatment. The secondary outcomes were adverse events and recurrence.
Data synthesis and Statistical analysisSex, adverse events, and the total number of patients with LGD or HGD/IMC are expressed as absolute numbers. Age, maximum BE length, and body mass index are presented as means with standard deviations. Statistical analyses of CE-IM, CE-D, and recurrence outcomes were performed using the Review Manager software ver. 5.4 (Cochrane). Heterogeneity was assessed using the statistical inconsistency index (I2). values of <30%, 30% to 60%, 61% to 75%, and >75% were considered low, moderate, high, and very high, respectively. The random-effects model was used for high/very high values, and the fixed-effects model was used for low/moderate values. These results were risk differences (RDs) with corresponding confidence intervals [CIs] (95% CI).
RESULTSResult of the literature searchA total of 11,107 studies were identified using this search strategy. A total of 4,827 duplicate articles were excluded. Evaluation of the titles and abstracts led to the selection of 14 studies that answered the clinical question and were analyzed in full text. Based on the inclusion and exclusion criteria, 10 were excluded for incomplete data and one for the analysis of another population profile. The remaining three studies were included in the final analysis. A schematic diagram of the identification and selection of the studies is shown in Figure 1.
Study characteristicsThree were retrospective cohort studies. Two were multicenter studies,20,21 and one was a single-center study.22 In one study, the number of pathologists was not mentioned,20 whereas in others, the participation of two independent pathologists was reported.21,22 However, all the included studies referenced pathologists specializing in the gastrointestinal tract. All evaluated patients with CE-IM and CE-D underwent a minimum follow-up of one year with surveillance endoscopy. Regarding the cryotherapy modality, two studies used liquid nitrogen,21,22 and one used nitrous oxide.20 Endoscopic examinations were performed using high-definition devices, chromoscopy, and protocolized biopsies (Table 1).20-22
Demographic profile and histological characteristicsA total of 627 patients were included in this analysis. Of these, 228 were in the cryotherapy group, and 399 underwent RFA. There was a predominance of overweight/obese men, with a mean age of over 60 years and a mean maximal BE length greater than 3 cm in both groups in all studies. Among those with LGD, 182 underwent RFA, and 79 underwent cryotherapy; among those with HGD/IMC, 217 received RFA and 149 received cryotherapy. Patients with visible lesions were evaluated for resection prior to ablative therapy. The detailed data are presented in Table 2.20-22
Technical differences in ablative modalitiesThe three included studies performed RFA using circumferential and sectorial catheters. The energy applied was 12 to 15 J/cm2 every 3 cm, in a downward manner, in the area to be treated. The coagulated tissue was scraped using a cap mounted on an endoscope. A second series of ablations was then performed. When the sectorial catheter was used, energy was applied twice before scraping, which was performed using the tip of the focal catheter.
Thota et al.22 and Fasullo et al.21 used a cryospray catheter passed through the endoscopeãs working channel, delivering liquid nitrogen at ã196 ô¤C to perform cryotherapy. A gastric decompression device was also required to remove the gas. Areas measuring 2 to 3 cm2 were progressively treated semicircularly until the entire BE was reached. Each site was frozen for 2 to 3 cycles of 20 to 30 seconds each, with at least 45 to 60 seconds between cycles to allow tissue thawing.
On the other hand, Agarwal et al.20 used a cryoballoon catheter. The device was passed through the working channel and positioned in the area to be treated to perform this procedure. Then, the nitrous oxide was released with inflation of the balloon and application of cryogen for 10 seconds, cooling the tissue to ã85 ô¤C. Areas 2 to 3 cm in diameter were treated until the entire segment of the BE was reached.
The number of sessions and interval between sessions for each ablative technique in each study are described in the supplementary material (Supplementary Table 1).20-22
Advantages and disadvantages of ablative modalitiesThe advantages of RFA are its greater availability, better direct visualization of the mucosa during the procedure, avoidance of overlapping treated areas, and better options in cases of altered gastric anatomy, such as gastric bypass, stomach stapling, and gastrojejunostomy, as there is no gas insufflation, which reduces the risk of perforation. Its disadvantages include a greater number of endoscopic intubations per session owing to the need to scrape the mucosa between ablations, the need to use sectorial catheters to approach residual areas, and the difficulty in passing the circumferential catheter (owing to its large diameter) in patients with stenosis.
Cryotherapy has the advantage of causing less pain after the procedure, is better applicable to patients with an irregular esophageal wall, and has the potential to reach a greater depth of the mucosa. Its disadvantages include the need to pass a probe for gastric decompression for devices that use nitrogen spray catheters, a longer procedure time during the treatment session owing to the need for freezing and thawing cycles, a greater potential risk of stenosis because it reaches a greater depth, and a lower availability of services.
Risk of bias and quality of studiesApplication of the ROBINS I tool identified two studies with a serious risk of bias, mainly due to confounding and patient selection bias,21,22 and one with a low risk of bias.20 A detailed description of the risk of bias is presented in Figure 2. The quality of evidence in the included studies was considered very low (Supplementary Table 2).
Adverse events and recurrenceRecurrence occurred in patients with at least 12 months of follow-up after CE-IM. A total of 186 patients from two studies were analyzed.21,22 There was no difference in the recurrence rate between the RFA and cryotherapy groups (RD, 0.09; 95% CI, ã0.02 to 0.19; p=0.12; I2=0%) (Fig. 5). GRADE analysis revealed a very low certainty of evidence. Adverse events were described in two studies.20,21 Because of their low occurrence, their values were expressed in absolute numbers (Table 3).20-22
DISCUSSIONWe present the first meta-analysis to compare RFA and cryotherapy for dysplastic BE with or without early esophageal neoplasia. This study showed that both were equally effective and that there was no difference in the recurrence rate of intestinal metaplasia. The number of adverse events was low in both groups.
This meta-analysis gathered the available scientific evidence to date. No randomized clinical trials have compared these two methods. Only four existing studies were cohort studies. Three studies were included in the meta-analysis. One study with 46 patients was not included because it evaluated efficacy using non-histological methods. The excluded studies used visual scoring to assess the replacement of BE mucosa with squamous mucosa during endoscopy after a single treatment session. Interestingly, this study found no significant difference in efficacy between the two methods.23
Only Thota et al.22 identified a difference in the CE-IM analysis (66.7 vs. 41.3%, p=0.002), favoring RFA. In this study, cryotherapy was the method of choice in cases where RFA was not feasible owing to an uneven surface, stenosis preventing passage of the RFA device, and IMC in the proximal esophagus without the possibility of resection. This method of choice is also suitable for patients with a bleeding diathesis. These baseline differences between the groups may have influenced the results.
Cryotherapy is a novel technique that has improved over the last several years owing to the increasing experience. Thus, older studies may underestimate the efficacy of this method compared to the effect. Newer cryotherapy devices offer numerous theoretical advantages, including more effective ablation over wider areas. This phenomenon may explain the results of the study by Thota et al.,22 which revealed a CE-IM of only 41.3% using data collected from 2006 to 2011, the oldest period among the included studies.
Differences were also observed among the cryotherapy modalities used. All modalities share the same principle of using freezeãthaw cycles, but the technique with the cryoballoon stood out compared to others. A study conducted by Canto et al.24 in 2018 revealed that cryoballoons can achieve a CE-IM of 88% and a CE-D of 95%. Previously published meta-analyses evaluating the efficacy of cryotherapy also pooled different modalities, as in the present study.12,13
Importantly, there is a need to maintain posttreatment surveillance for both ablative modalities because of the possibility of recurrence. Two studies evaluated these outcomes.21,22 Recurrence was defined as endoscopically visible or histological BE after CE-IM. These studies evaluated occurrence among patients with at least 12 months of follow-up. At the end of treatment, patients underwent surveillance endoscopy with biopsies every 3 to 6 months during the first year. Although there was a continuation of follow-up after the first year, there was no precise description of the total follow-up time.
A prospective study by Cotton et al.25 assessed long-term (four-year) outcomes and showed that most recurrences occurred in the first year after ablative therapy. This study also demonstrated that the probability of recurrence in the first year after CE-IM was higher than that in the following four years combined.
A comparative statistical analysis of adverse events was not performed because of a lack of data in one of the studies22 and the very low number of endpoints in the others.20,21 Studies with larger patients are required to evaluate this outcome accurately.
The lack of randomization and preferential assignment of patients to a particular therapy in all three studies may have influenced the results by promoting differences between groups. Thus, bias vulnerability, such as confounding factors and selection, can be identified. Agarwal et al. attempted to minimize the bias by conducting subgroup analyses and propensity score matching.20 After these adjustments, similar efficacy data were obtained for RFA and cryotherapy.
The patient profile in this meta-analysis was similar to that of the population with the highest prevalence of BE in real-world clinical practice. The representative patient population makes these data applicable in clinical practice and likely reproducible. However, the populations included in the studies were followed up at tertiary referral centers, which likely led to a referral bias. Importantly, leading gastroenterology and endoscopy societies uniformly recommend that EET be performed at centers with experience in endoscopic resection and ablation.26-28
The main strength of this meta-analysis was the inclusion of only comparative studies (cryotherapy vs. RFA) with histological evaluation by experts for diagnosis and effectiveness after treatment. Other relevant aspects include the determination of CE-IM and CE-D as primary outcomes because they are the most established measures of efficacy, the presence of a sample composed of a reasonable number of patients, the presence of two multicenter studies, and the exclusion of studies without adequate follow-up.
This meta-analysis had some limitations. All included studies were retrospective cohort studies; however, they were the only available evidence. The main outcomes showed high heterogeneity, which may have been secondary to methodological and clinical variability. This study was vulnerable to confounding factors and selection and referral biases. It was impossible to evaluate the efficacy adjusted for subgroups according to BE length or initial histological grade owing to the lack of data.
The choice of RFA or cryotherapy in treating dysplastic BE with or without early esophageal neoplasia should be made individually considering device availability, cost, personal experience, and patient preference; in terms of efficacy, both methods appear comparable. More studies, especially randomized clinical trials, are required to expand the available evidence to assist in clinical decision-making.
In conclusions, the available data suggest that cryotherapy and RFA are equally effective in achieving CE-IM and CE-D in patients with dysplastic BE with or without early esophageal neoplasia.
Supplementary MaterialSupplementary Table 2. Analysis of the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria. Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2023.065.
NOTESAuthor Contributions
Conceptualization: ILCG, DTHM, SBM, ASC, WMB, EGHM; Data curation: all authors; Formal Analysis: ILCG, IBR; Methodology: ILCG, SBM, ASC, WMB; Software: ILCG, BCMN, BSH, GHPO; Supervision: EGHM; Writingãoriginal draft: all authors; Writingãreview & editing: ILCG, DTHM, RPT, EGHM.
Fig.ô 1.Study selection flowchart according to Preferred Reporting Items for Systematic Reviews and Meta-analyses. 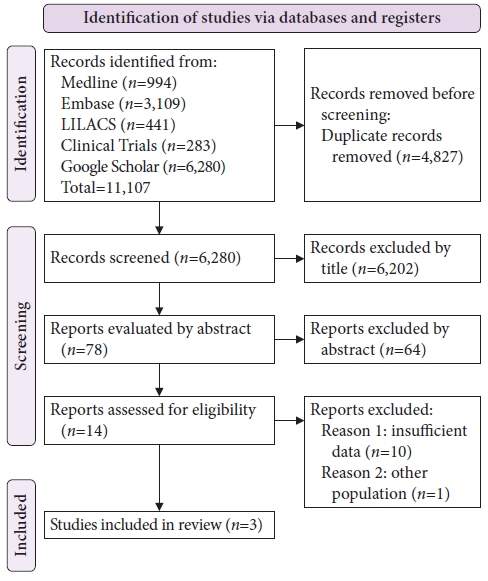
Fig.ô 3.Rate of complete eradication of intestinal metaplasia. M-H, Mantel-Haenszel test; CI, confidence interval; I2, heterogeneity. 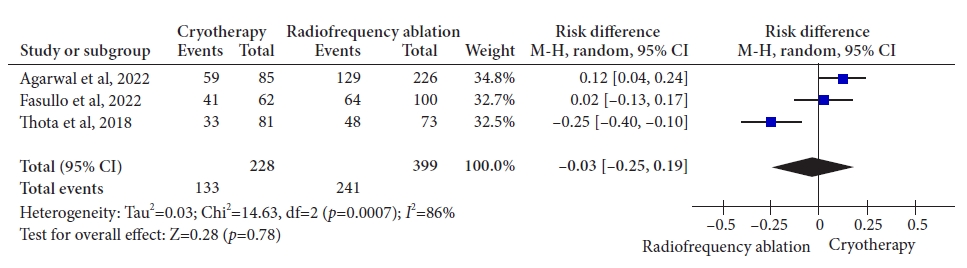
Fig.ô 4.Rate of complete eradication of dysplasia. M-H, Mantel-Haenszel test; CI, confidence interval; I2, heterogeneity. 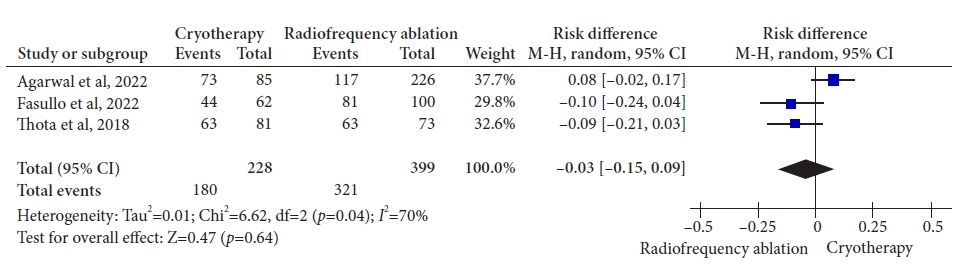
Tableô 1.Study characteristics
Tableô 2.Demographic profile and histological characteristics
REFERENCES1. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrettãs esophagus with dysplasia. N Engl J Med 2009;360:2277ã2288.
2. Garg S, Xie J, Inamdar S, et al. Spatial distribution of dysplasia in Barrettãs esophagus segments before and after endoscopic ablation therapy: a meta-analysis. Endoscopy 2021;53:6ã14.
3. de Matos MV, da Ponte-Neto AM, de Moura DTH, et al. Treatment of high-grade dysplasia and intramucosal carcinoma using radiofrequency ablation or endoscopic mucosal resection + radiofrequency ablation: meta-analysis and systematic review. World J Gastrointest Endosc 2019;11:239ã248.
4. Pouw RE, Klaver E, Phoa KN, et al. Radiofrequency ablation for low-grade dysplasia in Barrettãs esophagus: long-term outcome of a randomized trial. Gastrointest Endosc 2020;92:569ã574.
5. Sawas T, Alsawas M, Bazerbachi F, et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrettãs esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019;89:913ã925.
6. De Souza TF, Artifon EL, Mestieri LH, et al. Systematic review and meta-analysis of endoscopic ablative treatment of Barrettãs esophagus. Rev Gastroenterol Peru 2014;34:217ã224.
7. Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrettãs esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191ã198.
8. Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrettãs Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245ã1255.
9. Pandey G, Mulla M, Lewis WG, et al. Systematic review and meta-analysis of the effectiveness of radiofrequency ablation in low grade dysplastic Barrettãs esophagus. Endoscopy 2018;50:953ã960.
10. Wolfson P, Ho KMA, Wilson A, et al. Endoscopic eradication therapy for Barrettãs esophagus-related neoplasia: a final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc 2022;96:223ã233.
11. Barret M, Pioche M, Terris B, et al. Endoscopic radiofrequency ablation or surveillance in patients with Barrettãs oesophagus with confirmed low-grade dysplasia: a multicentre randomised trial. Gut 2021;70:1014ã1022.
12. Hamade N, Desai M, Thoguluva Chandrasekar V, et al. Efficacy of cryotherapy as first line therapy in patients with Barrettãs neoplasia: a systematic review and pooled analysis. Dis Esophagus 2019;32:doz040.
13. Tariq R, Enslin S, Hayat M, et al. Efficacy of cryotherapy as a primary endoscopic ablation modality for dysplastic Barrettãs esophagus and early esophageal neoplasia: a systematic review and meta-analysis. Cancer Control 2020;27:1073274820976668.
14. Visrodia K, Zakko L, Singh S, et al. Cryotherapy for persistent Barrettãs esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396ã1404.
15. Trindade AJ, Inamdar S, Kothari S, et al. Feasibility of liquid nitrogen cryotherapy after failed radiofrequency ablation for Barrettãs esophagus. Dig Endosc 2017;29:680ã685.
16. Solomon SS, Kothari S, Smallfield GB, et al. Liquid nitrogen spray cryotherapy is associated with less postprocedural pain than radiofrequency ablation in Barrettãs esophagus: a multicenter prospective study. J Clin Gastroenterol 2019;53:e84ãe90.
17. Canto MI, Trindade AJ, Abrams J, et al. Multifocal cryoballoon ablation for eradication of Barrettãs esophagus-related neoplasia: a prospective multicenter clinical trial. Am J Gastroenterol 2020;115:1879ã1890.
18. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 [Internet]. The Cochrane Collaboration; 2019 [cited 2023 Jan 31]. Available from: https://training.cochrane.org/handbook.
19. GRADEpro. Guideline Development Tool software [Internet]. McMaster University; 2023 [cited 2023 Jan 31]. Available from: https://gdt.gradepro.org/app/.
20. Agarwal S, Alshelleh M, Scott J, et al. Comparative outcomes of radiofrequency ablation and cryoballoon ablation in dysplastic Barrettãs esophagus: a propensity score-matched cohort study. Gastrointest Endosc 2022;95:422ã431.
21. Fasullo M, Shah T, Patel M, et al. Outcomes of radiofrequency ablation compared to liquid nitrogen spray cryotherapy for the eradication of dysplasia in Barrettãs esophagus. Dig Dis Sci 2022;67:2320ã2326.
22. Thota PN, Arora Z, Dumot JA, et al. Cryotherapy and radiofrequency ablation for eradication of Barrettãs esophagus with dysplasia or intramucosal cancer. Dig Dis Sci 2018;63:1311ã1319.
23. van Munster SN, Overwater A, Haidry R, et al. Focal cryoballoon versus radiofrequency ablation of dysplastic Barrettãs esophagus: impact on treatment response and postprocedural pain. Gastrointest Endosc 2018;88:795ã803.
24. Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrettãs esophagus (with video). Gastrointest Endosc 2018;88:438ã446.
25. Cotton CC, Wolf WA, Overholt BF, et al. Late recurrence of Barrettãs esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681ã688.
26. Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and management of Barrettãs esophagus: an updated ACG guideline. Am J Gastroenterol 2022;117:559ã587.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
