An Unusual Mimicker of a Pancreatic Pseudocyst
Article information
A 46-year-old woman without a significant history presented our emergency room with acute epigastric pain. Abdominal computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) demonstrated a well-defined 2.1-cm cystic lesion at the neck of the pancreas, without peripancreatic infiltration, that was connected to the pancreatic duct (Fig. 1A). Serum laboratory findings were as follows: amylase 142 U/L, lipase 149 U/L, carcinoembryonic antigen (CEA) 0.94 ng/mL, and cancer antigen 19-9 10.22 U/mL. Endoscopic ultrasound (EUS) demonstrated a distinct anechoic lesion connected to the main pancreatic duct (Fig. 1B). EUS-guided fine needle aspiration (EUS-FNA) retrieved clear cystic fluid with low viscosity. Histopathologic examination of the cystic fluid demonstrated ductal epithelial cells without a malignant component. Analysis of the cystic fluid showed CEA 10.45 ng/mL and amylase 126 IU/L, suggesting low potential for malignancy. Thus, the pancreatic cystic lesion was initially considered a pseudocyst. Follow-up CT performed after 3 months revealed a short stricture of the pancreatic duct with mild upstream dilatation around the cystic lesion. Endoscopic retrograde cholangiopancreatography demonstrated a connection of the cystic lesion (arrow) to the main pancreatic duct (Fig. 1C); a plastic stent was inserted to improve the stricture. During the 6-month follow-up period after initial presentation, the size of the cystic lesion did not dramatically decrease (Fig. 1D). Thereafter, the patient underwent central pancreatectomy. Histopathologic examination demonstrated a unilocular cyst containing serosanguineous fluid, and a surrounding solid component that was diagnosed as a Grade 2 pancreatic neuroendocrine tumor (pNET) (Fig. 2). Immunohistochemical staining showed positive results for CK, CD56, and synaptophysin.
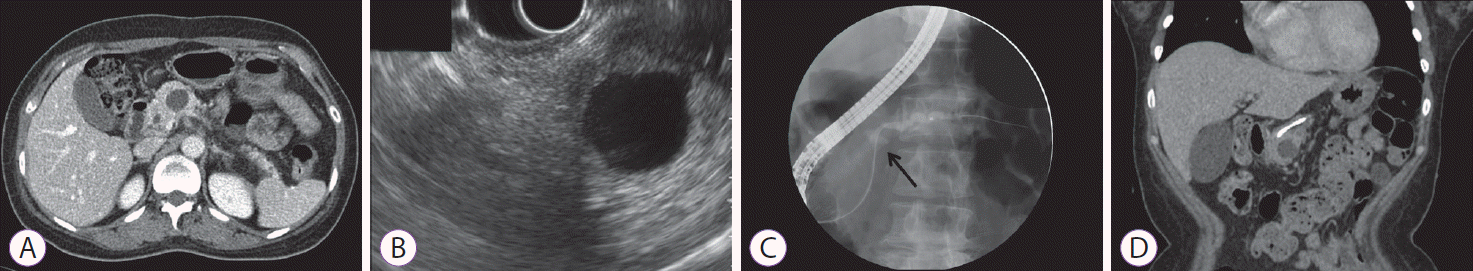
Imaging modalities for the pancreatic cystic lesion. (A) Abdominal computed tomography (CT) shows a well-defined cystic lesion measuring 2.1 cm at the neck of the pancreas, connected to the pancreatic duct. (B) Endoscopic ultrasound demonstrates an anechoic cystic lesion without solid content. (C) Pancreatography shows a connection of the cystic lesion (arrow) to the main pancreatic duct. (D) Follow-up CT shows no interval change in the size of the cystic lesion despite the long-lasting plastic stent.
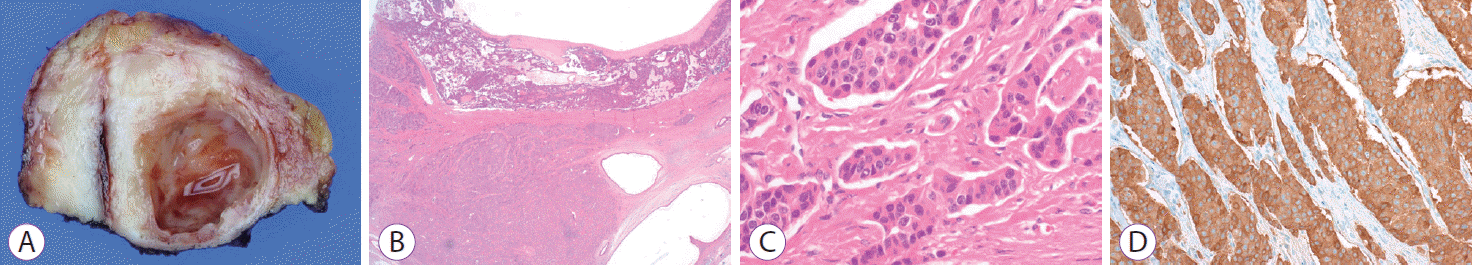
Histopathologic examination of the resected pancreatic cystic lesion. (A) Gross examination shows a unilocular cyst containing serosanguineous fluid. (B-D) The surrounding solid component diagnosed with a grade 2 neuroendocrine tumor (B, ×12, hematoxylin and eosin [H&E]; C, ×400, H&E; D, ×200, synaptophysin).
Pancreatic cystic lesion is a common incidental finding in clinical practice. CT, EUS-FNA, and MRCP are helpful for the differential diagnosis of pancreatic cystic lesion. However, if the diagnosis is uncertain, the clinical experience and decision of the treating physician could also be an important diagnostic tool.
Although pNETs are typically solid, approximately 10%–20% of them undergo cystic degeneration, resulting in the radiologic interpretation of a pseudocyst [1]. CT, MRCP, EUS, and EUS-FNA with cystic fluid analysis aid the diagnosis of cystic pNETs [2]. Although cross-sectional images and cystic fluid markers are strongly suggestive of a benign pseudocyst, a possibility of malignancy should be considered throughout. Surgical resection of primary pNETs is associated with improved survival for patients in all stages of cancer, and it should be considered in suitable candidates [3].
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
