INTRODUCTION
Cystic lymphangiomas are rare benign tumors of the lymphatic system. Ninety percentage of cystic lymphangiomas are found in the neck and axillary regions.1 Retroperitoneal lymphangiomas account for nearly 1% of all lymphangiomas.2 Although often asymptomatic, they can present with a palpable abdominal mass and nonspecific gastrointestinal symptoms such as abdominal pain and nausea/vomiting. They are often found incidentally during diagnostic procedures performed for unrelated clinical reasons.3 Approximately 90% of retroperitoneal lymphangiomas are diagnosed in the first 2 years of life; however, they can present at all ages and will often attain a large size prior to becoming symptomatic.4 These tumors rarely undergo spontaneous resolution and therefore treatment is usually recommended.5 Although rare in incidence, these lesions have been shown to be accurately diagnosed with endoscopic ultrasound guided fineneedle aspiration (EUS-FNA).6
CASE REPORT
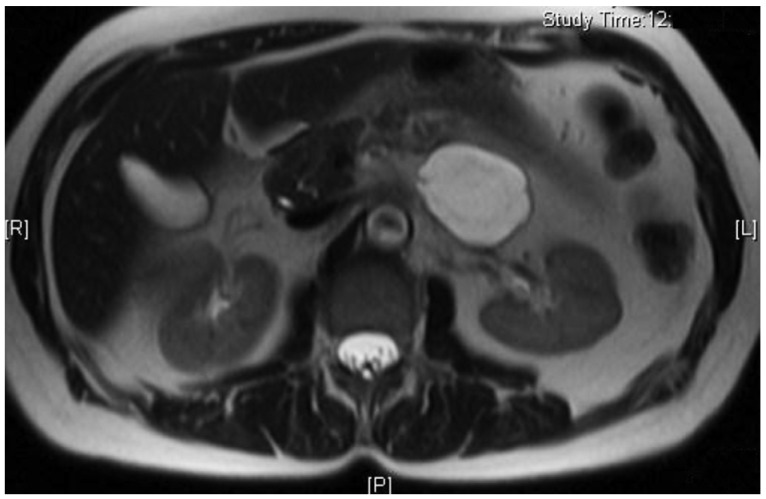
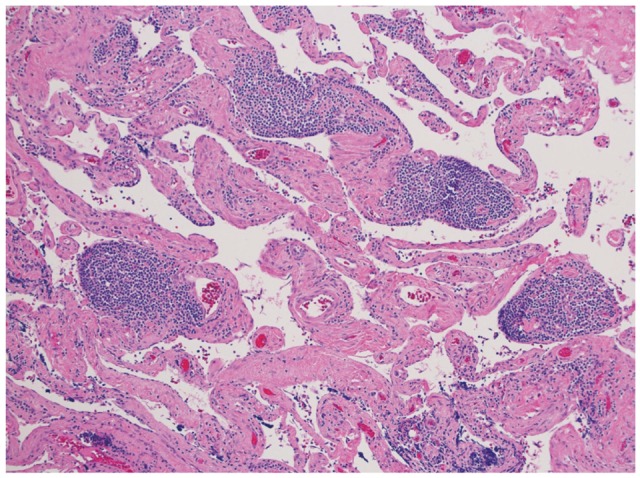
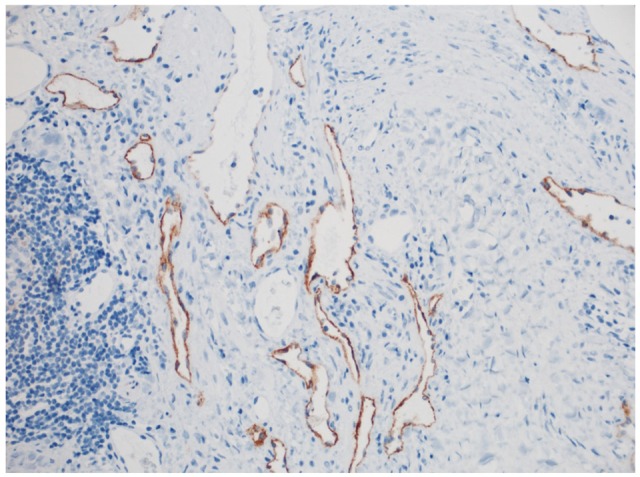
A 66-year-old female presented to her primary care physician for evaluation of 3 months of abdominal pain. Her pain was associated with heartburn, bloating, and indigestion and had been unresponsive to acid suppressing therapy. She denied any nausea or vomiting but did note occasional diarrhea. Further evaluation was performed with an abdominal ultrasound which revealed a 5 cm hypoechoic mass in the region of the tail of the pancreas. She underwent a magnetic resonance imaging (MRI) which confirmed the mass and noted it to be cystic in nature with multiple thin septations (Fig. 1). The patient was referred to gastroenterology and underwent EUS with FNA of the cystic lesion. EUS identified a 4.7├Ś3.3 cm cystic lesion with internal septations adjacent to the tail of the pancreas, but not within the pancreas itself (Fig. 2). White colored fluid was aspirated using a 19 gauge Cook Echotip FNA needle and initial fluid analysis was notable for lymphocytes. A fluid triglyceride level was noted to be elevated at 8,243 mg/dL. The patient was referred to surgery and underwent laparoscopic removal of the cystic lesion. Post resection, surgical pathology specimens were consistent with a cystic lymphangioma (Figs. 3, 4). At her 1 month postoperative visit, the patient noted significant improvement in her abdominal pain.
DISCUSSION
The differential diagnosis for a cystic lesion of the retroperitoneum is broad and includes both benign and malignant tumors including cystic mesothelioma, teratoma, undifferentiated sarcoma, malignant mesenchymoma, pancreatic pseudocyst, and lymphangioma.2 Retroperitoneal cystic lymphangiomas are rare tumors that are thought to arise due to an abnormal connection between the iliac and retroperitoneal lymphatic sacs and the venous system, leading to lymphatic stasis in the sacs.2 Retroperitoneal lymphangiomas are often classified into cystic and cavernous types.7 The cavernous type is usually patent to adjacent lymph flow and therefore compressible; whereas, cystic lymphangiomas are noncompressible and may be uniloculated or multiloculated.4 An additional third type, capillary lymphangioma, is rarely seen in retroperitoneal lymphangiomas. Preliminary imaging with ultrasound can be useful given that it can demonstrate the cystic nature of a lesion. Further imaging with computed tomography or MRI can help further classify cysts as unilocular or multilocular, assess the relationship of the lymphangiomas to adjacent organs, and further delineate the boundaries of the cyst.2 Most retroperitoneal lymphangiomas are diagnosed incidentally in asymptomatic patients, but complications such as severe abdominal pain, hemorrhage, infection, torsion, rupture, and obstruction can occur.8 A fluid that appears grossly chylous with a high triglyceride level is essentially diagnostic of a cystic lymphangioma.8 Surgical excision is considered to be the treatment of choice for cystic lymphangiomas given their potential to grow and develop complications.9 Alternative treatments in the form of aspiration, radiotherapy, and sclerotherapy have been reported with variable results.4
The role of EUS-FNA is less defined with respect to rare cystic diseases but has been evolving over the last decade. Just as EUS has become invaluable in pancreatic lesions, it is also beneficial in nonpancreatic tumors of the retroperitoneum. Imaging modalities can provide useful diagnostic information but cannot determine whether a lesion is benign or malignant. The ability for cell sampling with EUS-FNA makes it an important diagnostic modality in this regard and can help further guide subsequent therapeutic strategy. The performance characteristics of EUS-FNA have been shown to be quite good with a specificity of 100% and accuracy of 86% previously reported in the literature.10 The risks associated with EUS-FNA of these lesions are small, mainly bleeding, and infection. Tumor seeding or leakage of lymphatic fluid with EUS-FNA has been sparsely reported in the literature and substantial evidence for this complication is still lacking.11 Given the low risk nature, diagnostic accuracy, and ability for cell sampling, EUS-FNA should be considered a first line modality in the evaluation of these cystic lesions of the retroperitoneum.