AbstractSocial networks are useful in the study of relationships between individuals or entire populations, and the ties through which any given social unit connects. Those represent the convergence of the various social contacts of that unit. Consequently, the term "social networking service" (SNS) became extremely familiar. Similar to familiar SNSs, International Digestive Endoscopy Network (IDEN) 2014 was based on an international network composed of an impressive 2-day scientific program dealing with a variety of topics for gastrointestinal (GI) diseases, which connects physicians and researchers from all over the world. The scientific programs included live endoscopic demonstrations and provided cutting-edge information and practice tips as well as the latest advances concerning upper GI, lower GI, and pancreatobiliary endoscopy. IDEN 2014 featured American Society for Gastrointestinal Endoscopy-Korean Society of Gastrointestinal Endoscopy (ASGE-KSGE)-joint sessions prepared through cooperation between ASGE and KSGE. Furthermore, IDEN 2014 provided a special program for young scientists called the 'Asian Young Endoscopist Award Forum' to foster networks, with many young endoscopists from Asian countries taking an active interest and participation.
INTRODUCTIONA social networking service (SNS) is a platform to build social networks or social relations among people who share interests, activities, backgrounds, or real-life connections. SNSs consist of a representation of each user, his social links, and a variety of additional services. Most SNSs are web-based services that allow individuals to create a public profile, generate a list of users with whom to share connections, and view and cross the connections within the system, and they provide means for users to interact over the Internet such as e-mail and instant messaging. In addition to these common web-based features, social network sites are varied, and they incorporate new information and communication tools such as mobile connectivity, photo and video sharing, and blogging. Thus, SNSs allow users to share ideas, pictures, posts, activities, events, and interests with people in their network.
In scientific and medical fields, networking is also implicated in the core of contribution. For instance, given the functional interdependencies between molecular components, a disease is rarely the consequence of a single gene abnormality. Instead, a disease typically reflects the perturbations of intercalating intracellular and intercellular networks that influence the status of tissues or organs with accumulated dysfunction, or so-called systems biology relevant to clinical diseases.1 A network of disorders and disease genes linked by known disorder-gene associations offers a platform to explore a single graph-theoretic framework, indicating that the common genetic origin is practically implicated in many clinical diseases.2 These two examples stress the importance of "networks" in both science and medicine. As a result, the emerging tools of network medicine provide a platform to systematically explore both the molecular complexity of a particular disease and the molecular relationships among apparently distinct pathophenotypes. In searches for etiological pathogenic genes in our laboratory, we have obtained results from network analysis using next-generation proteomics indicating that radiation-induced intestinal damage is caused by oxidative stress and structural disruption (Fig. 1A). The gene expression patterns indicate that they are localized in the functional core of the network relevant to radiation-associated GI damage. Remarkable advances in endoscopic technology and the evolution of mechanic science are never missed with network sharing, by which International Digestive Endoscopy Network (IDEN) 2014 was held from May 30, 2014 to June 1, 2014 at the Sheraton Hotel in Seoul, Korea (Fig. 1B). This impressive 2-day scientific program dealt with a wide variety of basic concerns including upper gastrointestinal (GI), lower GI, and pancreaticobiliary endoscopy, and this year we added a network event named the Asian Young Endoscopist Award (AYEA) forum (Fig. 2). This award provides funding for endoscopic training to permit 28 active endoscopists from nine countries in this AYEA to acquire or develop new knowledge or technical skills regarding GI endoscopy. This newly acquired knowledge or endoscopic skill can then be used to improve patient care in the applicants' regions. In addition, we also held "Editor School III" to promote our society's journal, Clinical Endoscopy, during the AYEA forum. Furthermore, there were four American Society for Gastrointestinal Endoscopy-Korean Society of Gastrointestinal Endoscopy (ASGE-KSGE) joint sessions focused on "In vivo histologic image for GI diseases," "Safety issues in colonoscopy," "Can we resect and discard diminutive or small colorectal polyps?" and "Current issues of interventional endoscopic ultrasonography (EUS)." In this September issue of Clinical Endoscopy, we highlight cutting-edge review articles from contributing authors.
HOT TOPICS OF THE UPPER GI ENDOSCOPY SESSIONSAchalasia treatmentsAchalasia is an uncommon esophageal motility disorder typified by difficulty in swallowing, retention of food material, and secretion in the esophagus. This problem is caused by the degeneration of inhibitory ganglion cells in the myenteric plexus of the lower esophageal sphincter (LES), which results in increased basal tone and incomplete relaxation of the LES. The main treatment strategy is to weaken the LES barrier to prevent it from serving as a barrier to swallowed food moving toward the stomach. Traditionally, surgical treatment via Heller myotomy or endoscopic pneumatic balloon dilation has been the standard treatment for this condition.3 Recently, the highly promising endoscopic method of peroral endoscopic myotomy (POEM) was introduced. Botulinum toxin (Botox) injection can be used as an endoscopic pharmacologic treatment. In the IDEN session titled "From A to Z of achalasia treatment," these treatment options for the disease were thoroughly reviewed.
Impact of endoscopic treatment: pneumatic dilation and BotoxPneumatic balloon dilation is a simple method that can be applied as an initial treatment option. In this procedure, a large diameter pneumatic balloon of 30 to 40 mm in diameter is placed at the level of the LES and inflated to tear the sphincter muscle. Initial success rates after 1 year are reported to be excellent at 70% to 80%, but remission is maintained only in approximately 50% over a long-term follow-up period of more than 3 years. The major complication of this method is perforation, occurring in approximately 2% of procedures, which can be managed using conservative methods but may require emergency laparotomy.
Botox effectively inhibits acetylcholine release from nerve endings; thus, it can relieve unopposed LES contraction mediated by cholinergic nerves. In general, a total dose of 80 to 100 IU of Botox is endoscopically injected using a sclerotherapy needle in the four to eight directions of the LES. Prospective studies revealed that the response rates were approximately 80% at 1 month and approximately 60% by 12 months.3 In comparison with either pneumatic dilation or myotomy, Botox injection has a similar initial efficacy but carries the disadvantage of rapid deterioration at 6 to 12 months after injection. The effectiveness can be improved via multiple injections and in combination with pneumatic dilatation. Because Botox injection has the advantages of a simple technique and safety, it can be used in patients with significant comorbidities that prevent invasive procedures.
Laparoscopic myotomyHeller myotomy was introduced nearly a century ago in 1913.3 Currently, this procedure is usually performed using a laparoscopic approach with a minimal risk of complications. Antireflux measures are required to prevent severe acid reflux using a partial wrap via an anterior partial (Dor) or posterior partial (Toupet) method. High short-term success rates approaching 100% were reported, and durable long-term remission can also be achieved. A recent randomized trial comparing this method with balloon dilation reported comparable 2-year efficacy (86% for dilation and 90% for myotomy) and complication rates.4
POEM: an emerging concept for GI endoscopy and solving the challengePOEM is a recently developed and extremely promising method to treat achalasia. This endoscopic procedure uses a submucosal tunnel technique to reach the LES from an entry site at the mid-esophagus and finally disrupts the LES via myotomy. This technique was first introduced by Pasricha et al.,5 who reported the feasibility of the POEM procedure in a pig model. Subsequently, an initial human trial with excellent results was reported by Inoue et al.6 In Korea, an animal study was reported, and the initial human experiences also suggested the effectiveness of the method.7,8 After initial pilot studies investigating the strategy's safety and efficacy, a recent multicenter study reported the preliminary outcomes of 70 patients who underwent POEM at five centers in Europe and North America.9 Three months after POEM, 97% of patients exhibited symptom remission. The symptom remission rates were 89% and 82% at 6 and 12 months, respectively. Thus, POEM appears to be an effective treatment for achalasia for at least for 1 year, but further long-term follow-up research is needed. A recent observational study comparing laparoscopic Heller myotomy with POEM indicated that the techniques are associated with similar symptomatic and physiologic improvements.10 Outcome comparisons in long-term prospective randomized studies are needed.
Training for gastric endoscopic submucosal dissectionEndoscopic submucosal dissection (ESD) is a recommend treatment for early gastric cancer within the absolute or expanded indications carrying a minimal risk of lymph node metastasis. Although ESD is a minimally invasive treatment, it is mandatory to perform a curative treatment to achieve oncologically acceptable outcomes. Compared to endoscopic mucosal resection (EMR), ESD has higher en bloc and complete resection rates as well as higher complication rates. The expanded use of ESD permits the treatment of large lesions, those with ulcer findings, and those with an undifferentiated histology. Thus, rigorous training of endoscopists is necessary to achieve acceptable oncological outcomes and the safe use of the procedure.
How to improve the learning curve and teaching tips according to different knivesESD training programs consist of 1) observation and active assistance with ESD endoscopic procedures, 2) training using animal models, and 3) hands-on training under expert supervision. Endoscopists attempting ESD should be experts in endoscopic diagnosis for the proper selection of indicated lesions and in endoscopic procedures to prevent and provide adequate endoscopic treatment for complications such as bleeding and perforation. Fujishiro's11 review article teaching how to handle these complications is presented in this issue of Clinical Endoscopy. After the initial qualification, observation of ESD procedures using videos and participation in live demonstration conferences are needed. Then, actual observation of the ESD procedures of an expert is needed to learn basic techniques and handling of various situations encountered during the procedures.12,13 Knowledge about various types of endoscopic knives can improve endoscopists' technical competency and increase curative resection rates while decrease complications.
Training system for gastric ESDVarious regional efforts are ongoing for ESD training. Although gastric cancer is common in Eastern Asian countries, no authorized training protocol or certification is available.14 In Korea, annual live ESD demonstrations have been held since 2004 by KSGE, and the ESD study group of the KSGE has also held a nationwide hands-on course using ex vivo porcine models since 2007 and utilized in vivo models since 2009.12,15 Large institutions in Japan also provide their own training programs for ESD with highly effective outcomes under step-by-step training.16 Trainees should observe and attend as many cases of ESD as possible.
After the initial training, a learning curve must be completed to achieve competency in the use of ESD procedures. It was reported that approximately 30 to 40 cases of ESD must be performed before an endoscopist can competently and effectively perform ESD. For gastric ESD, antral lesions should be treated in up to 40 cases before attempting ESD at the mid and upper portions of the stomach.16 To improve the learning curve, a well-designed training program supervised by an expert is essential.
HOT TOPICS OF THE LOWER GI ENDOSCOPY SESSIONSNo more interval cancer in my practiceIt is hypothesized that most colorectal cancers (CRCs) diagnosed within a few years (3 to 5 years) after an index colonoscopy are the result of missed lesions or the development of new interval cancers. These tumors have been variously referred to as interval, missed, or postcolonoscopy CRCs. Colonoscopy in CRC screening is a golden standard test; however, it is not perfect. Although there is a recommended period for the next colonoscopy after an index colonoscopy, in real practice, clinicians may recommend a shorter duration.
My gosh! Have I missed it?The frequency of CRC after colonoscopy has been reported to vary from 2.9% to 7.9% depending on the institute. The reasons for interval CRCs are assumed to be incomplete bowel preparation, incomplete colonoscopy, missed lesions, incomplete polypectomy, and rapid tumor progression. According to a meta-analysis by Singh et al.,17 these cancers were 2.4-fold more likely to arise in the proximal colon (6.5%) than in the distal colon (2.9%). Patients with interval CRCs were older (age, >65 to 70 years vs. <65 to 70 years; odds ratio [OR], 1.15; 95% confidence interval [CI], 1.02 to 1.30), exhibited more comorbidities (high Charlson comorbidity index: OR, 2.00; 95% CI, 1.77 to 2.27), and more commonly had diverticular disease (OR, 4.25; 95% CI, 2.58 to 7.00). Many efforts have been made to prevent interval CRCs. Patients with a strong family history of CRC should undergo earlier and more frequent colonoscopic screenings or surveillance. Even in the best of hands, colonoscopy does not prevent all interval CRCs. Efforts should be made to improve the quality indicators of colonoscopy such as adenoma detection rates and withdrawal time.
Bowel preparation: impossible to be overemphasizedAn ideal bowel preparation would be simple, inexpensive, and palatable while achieving rapid and reliable bowel cleansing without adversely affecting the appearance of the colonic mucosa. However, there is currently no ideal bowel preparation technique. Split dosing is emphasized. Although many regimens have been developed, the use of 4 L of polyethylene glycol appears to be optimal with regard to cost, safety, and bowel cleanliness.
Keys to detecting early colorectal neoplasiaDetecting flat and depressed lesions during colonoscopy is difficult. Professor Hiroshi Kashida demonstrated some keys to detecting "difficult" lesions during colonoscopy. Although the fecal occult blood test (FOBT) is used globally as a screening test for CRC, it is associated with many false-positive and false-negative outcomes. Full colonoscopy should be performed once, even if the FOBT remains negative after repeated testing and the patient has never undergone colonoscopy. Endoscopists need to recognize depressed or flat lesions and understand their endoscopic features. For good bowel preparation, patients should take simethicone, a bubble-dissolving agent, while taking the cleanser. The endoscopist should wash off all stool residues during the observation. If a lesion is suspected during colonoscopy, then image-enhanced endoscopic techniques including narrow band imaging (NBI) and chromoendoscopy are recommended to delineate the lesion. In addition, it is noted that saline injection into the submucosal layer is helpful in delineating the lesion.
Strategy for incompletely resected colorectal neoplasmProfessor Bong Min Ko stated that interval CRCs can result from an incompletely resected lesion, either a cancer or an adenoma. It has been estimated that incompletely resected lesions during colonoscopy might explain 10% to 27% of observed interval CRCs. In his experience, larger polyps were more likely to be incompletely resected than smaller polyps. Ko generally performs additional maneuvers such as use of NBI or magnification to delineate polyp margins and assess margins after resection. In some cases, adjunctive ablation of the margins after resection of large polyps can be useful to assure complete removal.
Colorectal ESD: lessons from mastersESD of colorectal neoplasms is a challenging technique for most colonoscopists. Colorectal ESD has higher perforation rates, a longer procedure time, and greater learning difficulties than colorectal EMR. In some cases, piecemeal EMR is more effective than ESD.
Current indications and clinical outcomesThe indications for colorectal ESD as recommended by the Japanese Colorectal ESD Standardization Implementation Working Group are as follows: 1) lesions difficult to remove en bloc using a snare EMR because of their size, such as nongranular lateral spreading tumors (particularly pseudo-depressed type), lesions exhibiting a type VI pit pattern, and protruding-type large lesions suspected to be carcinoma; 2) lesions with fibrosis because of biopsy or peristalsis; 3) sporadic localized lesions in patients with chronic inflammation such as ulcerative colitis; and 4) local residual carcinoma after EMR.18 A systematic review by Repici et al.19 reported a histologically verified complete R0 resection rate of 88%. The R0 resection rate differed significantly between carcinoid and noncarcinoid series (93% vs. 87%) and between Asian and European series (93% vs. 87%). The rate of endoscopically complete resection was 96%. The bleeding and perforation rates per lesion were 2% and 4%, respectively. The surgical intervention rate because of an ESD-related complication was 1%. The local recurrence rate after colorectal ESD was 0.07% during a median follow-up period of 22 months.19
Techniques for safer and more rapid dissection: show me the secretsFujishiro20 reported his experiences regarding colorectal ESD techniques. A slim, single-channel, high-definition endoscope with a water-jet system and a high-frequency generator is helpful. The transparent attachment is fitted on the tip of the endoscope mainly to obtain a constant endoscopic view and create tension on the connective tissue for the submucosal dissection. CO2 insufflation is also preferable to relieve patients' discomfort during and after ESD. Retroflex positioning of the endoscope is usually used, if possible, in cutting a distal part. Dissection of the submucosal layer beneath the lesion should be performed to control bleeding during ESD. It is better to start cutting from an opposite section of the ground as well as a distal section or place the lesion on an opposite part of the ground if the patient's body positions are changeable.20 Additionally, the counter traction method reported by Oyama21 is helpful for submucosal dissection of lesions located in the rectum.
How to avoid and manage colorectal ESD complicationsColorectal ESD-related perforation rates have been reported to range 1.4% to 10.0%, and bleeding rates have ranged 1.2% to 2%. There are two types of perforation. One is endoscopically proven, and the other is radiologically proven. Risk factors for colorectal ESD-related perforations are large size, lesions in the cecum, performance by inexperienced endoscopists, and fibrosis. The use of hyaluronic acid as a submucosal cushion fluid is a protective factor against perforations. Pull-type knives such as the hook knife are useful in reducing the risk of perforation in the cecum. Surgery remains indicated for large perforations, generalized peritonitis, ongoing sepsis, aggravating peritonitis, and concomitant pathology such as advanced neoplasms that are difficult to resect endoscopically.22 Tight and close endoscopic clipping is especially useful in the closure of small perforations, which are commonly observed after colorectal ESD. There are two types of bleeding: immediate and delayed. Immediate bleeding occurs when submucosal vessels are cut without sufficient coagulation. Delayed bleeding occurs via the rupture of exposed vessels after completion of the ESD procedure. If vessel rupture is suspected, then ruptured vessels can be coagulated using hemostatic forceps or argon plasma coagulation. If the vessels are large, then clipping the vessels is a useful method to prevent delayed bleeding. Rarely, angiographic embolization is needed for bleeding control. Clipping is a key element for medical management of both perforation and bleeding.
HOT TOPICS OF THE PANCREATICOBILIARY SESSIONSIn IDEN 2014, there were seven pancreatobiliary sessions that were extremely informative and updated. These sessions were as follows: 1) endoscopic management of idiopathic recurrent pancreatitis; 2) new horizons for the management of challenging bile duct stones; 3) premalignant or early cancerous lesions in biliopancreatic trees; 4) pearls for endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA); 5) mucinous neoplasms of biliopancreatic trees; 6) current update for stenting for biliary strictures; and 7) new endoscopic diagnostic and therapeutic procedures for biliopancreatic lesions. In this section, each of the sessions will be summarized briefly.
Endoscopic management of idiopathic recurrent pancreatitisWhat are the differences between Asian and Western countries?This session dealt with the differences between Asian and Western countries concerning the management of idiopathic recurrent acute pancreatitis (IRAP). Sphincter of Oddi dysfunction (SOD) is the most common etiology of IRAP, and pancreas divisum with genetic mutation may be important in Western countries. However, in Asian countries, sphincter of Oddi manometry (SOM) is not frequently performed, and biliary microlithiasis is a more common cause of IRAP. EUS is considered the first-line examination technique in both Asian and Western countries. After negative EUS, secretin-enhanced magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography (ERCP) with SOM are the possible next steps in Western countries, whereas ERCP with intraductal ultrasonography (IDUS) or empirical endoscopic sphincterotomy (EST) is usually performed in Korea.
Endoscopic evaluation including EUS/IDUS and juice aspirationIn patients with IRAP, the role of noninvasive endoscopic diagnostic modalities such as EUS is increasing, and using this noninvasive technique, the causes of IRAP can be identified in approximately 70% of patients. However, ERCP with SOM has some role in identifying the causes of IRAP associated with SOD.
Motility disorders of SOD: evaluation and therapeuticsSOM is recommended in patients suspected of having type II or III SOD. However, SOM has never been demonstrated to predict the outcome of sphincterotomy in patients with type III SOD. Placebo effects are likely strong. Thus, the current practice of performing ERCP in these patients, with or without sphincterotomy and with or without SOM, is not supported by the evidence. Recently, a multicenter study was conducted to determine the effectiveness and safety of EST compared with sham treatment in adult patients with unexplained post-cholecystectomy pain. In patients with abdominal pain after cholecystectomy who underwent ERCP with SOM, sphincterotomy did not reduce disability caused by pain versus sham treatment. These findings do not support the use of ERCP and sphincterotomy for these patients.
Endoscopic treatment including empirical sphincterotomyIRAP requires an extensive evaluation to identify the possible causes. The treatment of patients with IRAP is aimed at specific etiologies. Endoscopic therapy with sphincterotomy or/and stenting for microlithiasis, SOD, and pancreas divisum is the treatment of choice.
New horizons for the management of challenging bile duct stonesEndoscopic large-balloon dilation combined with ESTThis session dealt with the indications, contraindications, and safety of endoscopic large-balloon dilation (EPLBD). EPLBD has been substituted for conventional methods such as full EST and mechanical lithotripsy to remove large and difficult bile duct stones. However, EPLBD also carries the possible serious complication of perforation. Patients targeted for EPLBD are those who already have a dilated common bile duct (CBD). Patients with distal CBD strictures because repeated cholangitis should be excluded from this procedure because of the possibility of perforation. Partial EST is preferred because it minimizes large vessel injury and perforation in comparison to full EST. Any marked resistance during balloon inflation is a relative contraindication for continued ballooning.
Endoscopic stenting and pharmacologic therapyAlternative methods for removing difficult CBD stones include electrohydraulic lithotripsy, extracorporeal shockwave lithotripsy, and laser lithotripsy. Temporary biliary stenting for challenging bile duct stones may prevent incarceration of the stone at the ampulla, provide good drainage of bile, and serve as a bridge therapy to secondary interventions. Combinations with temporary stenting and oral dissolution agents such as ursodeoxycholic acid and terpene preparations were proposed as treatments for challenging stones. However, the addition of choleretic agents did not result in a significant difference in stone size or the rate of successful duct clearance.
Stones in patients with surgically altered anatomiesIt has been challenging to perform conventional ERCP in patients with surgically altered GI anatomies. With the recent development of device-assisted enteroscopy (DAE), deep and even complete enteroscopy has become possible. At present, double-balloon enteroscopy, single-balloon enteroscopy, and spiral enteroscopy are globally available. The cap-assisted ERCP method can be considered a primary approach in patients with surgically altered anatomies including Billroth-II anatomy and Roux-en-Y anastomosis. Although DAE is an increasingly feasible alternative to ERCP for Roux-en-Y anastomosis, further optimization of the procedure is necessary to make DAE-ERCP the first method of choice in such patients.
Premalignant or early cancerous lesions in biliopancreatic treesEndoscopic diagnosis of premalignant or early cancerous biliary lesionsIDUS has been a useful diagnostic tool with extremely low adverse event rates for patients with equivocal findings on cholangiograms, as well as in the evaluation of indeterminate biliary strictures. Direct peroral cholangioscopy (POC) permits direct visualization of the biliary tree for diagnostic procedures and provides endoscopic guidance for therapeutic interventions. Direct POC allows the easy application of NBI to evaluate indeterminate biliary strictures.
Early diagnosis of pancreatic lesions: endoscopic approachEUS is useful for detecting small pancreatic masses, safely acquiring tissue, differentiating solid and cystic lesions, estimating the malignant potential for pancreatic cysts, and facilitating the differential diagnosis of small solid masses by applying contrast enhancement.
Endoscopic ablation therapyThe most exciting endoscopic ablative modality appears to be intraductal radiofrequency ablation using the Habib catheter and device. This technique is evolving and may provide a curative therapy with further improvement coupled with the early diagnosis of bile duct cancer.
Pearls for EUS-FNAFine needle biopsy: should this be the first choice?Usually, a cytological sample is sufficient to establish a diagnosis, but certain diseases such as lymphoma, stromal tumors, and autoimmune pancreatitis may require tissue core for a definite diagnosis. Therefore, EUS-fine needle biopsy can be recommended as a supplementary technique. However, needle stiffness is a major obstacle to overcome for transduodenal approaches for harvesting core tissue. Thus, needle selection should be based on many aspects such as the location and characteristics of the lesion and the suspected clinical diagnosis.23
Practical tips for the best resultCombined cytological-histological analysis improves the diagnostic accuracy compared with cytological analysis alone. Rapid on-site evaluation of the specimen with two-way communication is the most important factor to improve specimen adequacy and diagnostic accuracy. Communication between the endosonographer and cytopathologist is extremely important to minimize false-positive and false-negative results.
Neoplasia in chronic pancreatitis: how to maximize the yield of EUS-FNAWhen performing EUS-FNA, identifying neoplasia in the setting of chronic pancreatitis can be technically challenging. This session provided a concise update and offered piratical tips for improving the diagnostic yield of EUS-FNA when sampling solid pancreatic masses in the setting of chronic pancreatitis.24 Elastography and contrast-enhanced EUS were introduced, and the preliminary data suggested that these techniques may improve the ability to differentiate neoplasms from chronic pancreatitis. Performing the fanning method during FNA, utilizing rapid on-site evaluation, performing an adequate number of FNA passes, and procuring additional specimens for ancillary studies are critical steps.
Beyond solid pancreatic mass lesions: liver, lymphoma, sarcoidosis, and othersEUS can visualize the posterior mediastinum and tissues in the broad abdominal cavity such as adrenal gland, liver, spleen, gallbladder, bile duct, and intra-abdominal lymph nodes. In addition, the use of a large-gauge needle enables the collection of a histologic sample.
Mucinous neoplasms of biliiopancreatic treesNew classificationThis session introduced the classification of intraductal papillary neoplasms of the bile duct (IPN-Bs). An IPN-B may be the biliary counterpart of pancreatic intraductal papillary mucinous neoplasms (IPMNs). In parallel to pancreatic IPMNs, IPN-Bs histologically exhibit four phenotypes: pancreatobiliary, intestinal, gastric, and oncocytic.
Endoscopic evaluation of IPMNs of the pancreasAs multidetector computed tomography and magnetic resonance image provide good images, routine ERCP is not recommended. Precise pancreatograms and cytological assessment of the pancreatic juice are required in some patients, and IDUS and per oral pancreatoscopy may be useful to determine the resection margin in patients with main duct IPMNs. EUS often provides useful information for the differential diagnosis and evaluation of the malignant grade.
Endoscopic evaluation of IPMNs of the bile ductA dilated biliary system is featured as a typical imaging finding of IPN-Bs. IDUS is an effective tool for detecting biliary tumorous lesions during ERCP. Direct POC can visualize the papillary projection, which is a typical finding of IPN-Bs. Tissue sampling is possible, and NBI is a good modality to diagnose the cancer in IPN-Bs.
Are IPMNs of the pancreas and bile duct really the same disease?This session provided a concise update review about the pathologic and clinical characteristics of IPMNs of the pancreas and bile duct. The presenter could not determine whether IPMNs of the pancreas and bile duct represent the same disease, but IPN-Bs and pancreatic IPMNs share common histologic features and biological behaviors.
Current update for stenting for biliary stricturesTrial to longer stent patency: to break the limitationThis session introduced a strategy for developing superior self-expanding metal stents (SEMSs). The main cause of recurrent biliary obstruction in uncovered SEMSs was tumor/tissue ingrowth via the stent mesh. Uncovered metal stent placement with chemotherapy was reported as effective in prolonging stent patency in pancreatobiliary malignancies. The most important factor for prolonging stent patency is preventing both tumor ingrowth and migration. Covered SEMSs with a low incidence of migration may be ideal. This session introduced some special SEMSs expected to exhibit prolonged patency such as larger diameter metal stent and covered SEMSs with antireflux valves.
Bilateral stenting in malignant hilar obstructionThe strategy of palliative treatment differs according to the location or level of the malignant biliary stricture.25 There is significant controversy concerning whether single or multiple stents (unilateral vs. bilateral) should be inserted for Bismuth type II or higher tumors. The Asia-Pacific Working Group for hepatobiliary cancers stated that palliative stenting in patients with hilar cholangiocarcinoma should aim to achieve adequate drainage of at least 50% of the total liver volume, irrespective of whether unilateral, bilateral, or multisegmental stenting is used. The Asia-Pacific working group suggested that bilateral or multiple drainage is preferred in Bismuth type II tumors, whereas bilateral or multisegmental drainage via the percutaneous approach is preferred in Bismuth type III/IV tumors.
Anastomotic strictures after liver transplantationThis session massively reviewed the efficacy of plastic stents and SEMSs in the treatment of anastomotic strictures arising after liver transplantation. Eight studies with a total of 448 patients used plastic stents and reported a mean stricture resolution rate of 84.5% (range, 63% to 100%), which was comparable to eight prior studies involving a total of 180 patients who received metal stents that reported a mean stricture resolution rate of 75% (range, 53% to 94%). In conclusion, currently available SEMSs should not be offered for the management of anastomotic biliary strictures developing after liver transplantation.
Preoperative drainage for malignant biliary strictures: is it time for SEMSs?From the pathophysiological point of view, it is unquestionable that palliation of jaundice will be beneficial to patients awaiting surgery. The problem is that preoperative drainage often results in increased morbidity, which will affect surgical outcomes. The use of a better, wider lumen SEMS may dramatically change the outcome of preoperative biliary drainage. However, randomized controlled trials are needed to demonstrate this effect.
New endoscopic diagnostic and therapeutic procedures for biliopancreatic lesionsContrast-enhanced endoscopic ultrasonographyThis session reviewed the technique and role of contrast-enhanced endoscopic ultrasonography (CE-EUS). By eliminating Doppler-related artifacts, CE-EUS allows visualization of the microvasculature and parenchymal perfusion, which leads to both improved characterization of EUS-detected lesions and the identification of small tumors, as well as permits the estimation of malignant potential.
Enteroscopy for biliopancreatic lesionsERCP in patients with surgically altered anatomies is always challenging. In particular, the presence of a Roux-en-Y anastomosis has been considered to preclude endoscopic access for diagnostic and therapeutic ERCP because the long efferent and afferent limbs must be traversed to reach the major papilla or anastomotic site. This session reviewed the techniques of balloon enteroscopy-assisted ERCP in patients with surgically altered anatomies. Balloon enteroscopy-assisted ERCP appears to be a promising method for performing therapeutic ERCP in patients with surgically altered anatomies, though further development of accessories for use in balloon enteroscopic systems is needed.
EUS-guided treatment beyond drainage: hemostasis, anastomosis, and othersNew innovative EUS-guided techniques beyond drainage are currently being investigated. The presentation included celiac plexus block and neurolysis, several drainage methods (pancreatic fluid collection, abscess, and gallbladder), pancreatic necrosectomy, and oncologic interventions.26
HOT TOPICS OF THE ASGE-KSGE SESSIONSIn vivo histologic images for GI diseaseThere are many methods for the in vivo examination of the GI mucosa to differentiate benign and malignant histologic diagnosis. The most commonly utilized technologies feature image-enhanced endoscopy with/without magnification, including NBI, autofluorescence imaging, I-scans, and Fujinon intelligent chromoendoscopy. In the West, the use of confocal laser endomicroscopy (CLE) is expanding continuously. Probe-based CLE (pCLE) significantly improved the detection rate of neoplasia in Barrett's esophagus. It can be also used for the differentiation of adenoma from nonadenomatous polyps in the colon. In patients with inflammatory bowel disease, pCLE can be used for the early detection of dysplasia or disease relapse. Doctor Seok-Hyun Yun from Harvard Medical School in the United States reported the use of cellular-resolution fluorescence endomicroscopy in the mouse colon. He predicted that high-resolution optical endomicroscopy will have increasing application in studies of GI disease in animal models. Ko et al.27 from CHA Bundang Medical Center, CHA University in Korea discussed molecular imaging and submitted a review paper titled "Molecular imaging for theranostics in gastroenterology: one stone to kill two birds" for this issue of Clinical Endoscopy.27
Can we resect and discard diminutive or small colorectal polyps?The resect-and-discard strategy for small polyps was proposed by ASGE recently for cost reduction. However, there remain debates concerning its use. Although the technology of colonoscopy has advanced enormously, its accuracy of histologic diagnosis remains less than 100%. Doctor Hirishi Kashida from Japan emphasized that sufficient education and good techniques are prerequisite for real-time optical diagnosis and a margin-free resection to avoid potential risks of the resect-and-discard strategy. Doctor Yun Kyung Kang gave a speech from the pathologist's point of view. Her review article regarding this topic is published in this issue of Clinical Endoscopy.28
Safety issues in colonoscopySedation, the prevention and management of complications, and colonoscopy for patients with risks, including the elderly and debilitated patients receiving antiplatelet/anticoagulant therapy, were topics that were discussed in this session.
For safe, comfortable colonoscopy, moderate sedation is needed. Adequately trained endoscopists can perform a safe, high-quality examination while administering and supervising sedation.29 Propofol is administered by anesthesia professionals in the United States. However, endoscopist-directed propofol sedation is safe according to many studies. For patients who failed colonoscopy with standard sedation, monitored anesthesia care, in which the anesthetist administers propofol for deep sedation, is helpful. Sedation must be individualized. Patient characteristics associated with difficulty in sedation include anxiety, psychiatric disease, alcohol or drug abuse, narcotic usage, benzodiazepine usage, and prior difficulty with sedation.
For safe colonoscopic procedures, an excellent bowel preparation is essential. To prevent bleeding and perforation during endoscopic resection, the submucosa should be expanded maximally. Careful examination is critical to prevent local recurrence or interval cancer development. For the management of acute perforation, immediate suctioning of air and excessive fluid in the lumen is the first step. The defects should be closed with clips, followed by adequate care of pneumoperitoneum. Finally, the general surgical team should be notified for a team approach.
Currently, colonoscopy is frequently performed for elderly or debilitated patients. Colonoscopic complications are more common in elderly patients, including high rates of perforation and cardiovascular complications such as hypotension, arrhythmia, and hypoxia. Colonoscopy in the elderly is challenging, and adequate preparation and intensive monitoring are indispensible.
Endoscopic polypectomy is a high-risk procedure. If the patient is receiving antithrombotic therapy, then the urgency of the procedure and the risk of bleeding should be considered. The risk of bleeding or thromboembolic events should be considered before decision making concerning the discontinuation of antiplatelets/anticoagulants. Consultation to relevant physicians is recommended before discontinuing any antithrombotic agent.
Current issues of interventional EUSEUS-guided interventional procedures have proven extremely safe and effective. Presently, EUS-guided drainage procedures are frequently performed, and their targets are the gallbladder, biliary tree, pancreatic duct, pseudocysts, and walled-off necrosis. A review article containing details about the drainage of pseudocysts and walled-off necrosis can be found in this issue of Clinical Endoscopy.30
CONCLUSIONSThis issue of Clinical Endoscopy provides cutting-edge review articles presented during IDEN 2014. IDEN 2014 was an extremely informative and reinforcing meeting for both young endoscopists and experts. The scientific program of IDEN 2014 is provided as Supplementary Fig. 1. During this successful conference, every participant was able to update his or her knowledge and enhance his or her international network in the field of endoscopy. This year's IDEN was especially meaningful because of the ASGE-KAGE joint sessions, during which common and different opinions on interesting topics were exchanged. KSGE will continue its efforts to share comprehensive knowledge with other societies around the world.
References1. Barab├Īsi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet 2011;12:56ŌĆō68. 21164525.
2. Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barab├Īsi AL. The human disease network. Proc Natl Acad Sci U S A 2007;104:8685ŌĆō8690. 17502601.
4. Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807ŌĆō1816. 21561346.
5. Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761ŌĆō764. 17703382.
6. Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265ŌĆō271. 20354937.
7. Bang BW, Choi YC, Kim HG, et al. Peroral endoscopic myotomy for treating achalasia in an animal model: a feasibility study. Clin Endosc 2013;46:54ŌĆō58. 23423311.
8. Lee BH, Shim KY, Hong SJ, et al. Peroral endoscopic myotomy for treatment of achalasia: initial results of a Korean study. Clin Endosc 2013;46:161ŌĆō167. 23614126.
9. Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013;145:309ŌĆō311. 23665071.
10. Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg 2014;259:1098ŌĆō1103. 24169175.
11. Saito I, Tsuji Y, Sakaguchi Y, et al. Complications related to gastric endoscopic submucosal dissection and their managements. Clin Endosc 2014;47:398ŌĆō403.
12. Bok GH, Cho JY. ESD hands-on course using ex vivo and in vivo models in South Korea. Clin Endosc 2012;45:358ŌĆō361. 23251882.
13. Toyonaga T, Nishino E, Man IM, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc 2012;45:362ŌĆō374. 23251883.
14. Uedo N, Jung HY, Fujishiro M, et al. Current situation of endoscopic submucosal dissection for superficial neoplasms in the upper digestive tract in East Asian countries: a questionnaire survey. Dig Endosc 2012;24(Suppl 1):124ŌĆō128. 22533767.
15. Kim EY, Jeon SW, Kim GH. Chicken soup for teaching and learning ESD. World J Gastroenterol 2011;17:2618ŌĆō2622. 21677829.
16. Oda I, Odagaki T, Suzuki H, Nonaka S, Yoshinaga S. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24(Suppl 1):129ŌĆō132. 22533768.
17. Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:1375ŌĆō1389. 24957158.
18. Tanaka S, Terasaki M, Kanao H, Oka S, Chayama K. Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc 2012;24(Suppl 1):73ŌĆō79. 22533757.
19. Repici A, Hassan C, De Paula Pessoa D, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy 2012;44:137ŌĆō150. 22271024.
20. Fujishiro M. Endoscopic submucosal dissection for colorectal neoplasms. World J Gastrointest Endosc 2009;1:32ŌĆō38. 21160648.
21. Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375ŌĆō378. 23251884.
22. Raju GS, Saito Y, Matsuda T, Kaltenbach T, Soetikno R. Endoscopic management of colonoscopic perforations (with videos). Gastrointest Endosc 2011;74:1380ŌĆō1388. 22136781.
23. Kim EY. Fine-needle biopsy: should this be the first choice in endoscopic ultrasound-guided tissue acquisition? Clin Endosc 2014;47:425ŌĆō428.
24. Bang JY, Varadarajulu S. Neoplasia in chronic pancreatitis: how to maximize the yield of endoscopic ultrasound-guided fine needle aspiration. Clin Endosc 2014;47:420ŌĆō424.
25. Lee TH, Moon JH, Park SH. Bilateral metallic stenting in malignant hilar obstruction. Clin Endosc 2014;47:440ŌĆō446.
26. Widmer JL, Kahaleh M. Endoscopic ultrasound-guided treatment beyond drainage: hemostasis, anastomosis, and others. Clin Endosc 2014;47:432ŌĆō439.
27. Ko KH, Kwon CI, Park JM, Lee HG, Han NY, Hahm KB. Molecular imaging for theranostics in gastroenterology: one stone to kill two birds. Clin Endosc 2014;47:383ŌĆō388.
28. Kang YK. Diminutive and small colorectal polyps: the pathologist's perspective. Clin Endosc 2014;47:404ŌĆō408.
Fig.┬Ā1Network, a core keyword for advancement. In basic science, network research has emerged as an essential component of systems biology. (A) For example, network result was drawn from label free quantification study to pull out core pathogenic explanation of radiation-induced enteropathy. (B) International Digestive Endoscopy Network 2014 also sought to tighten international network in gastrointestinal endoscopy. 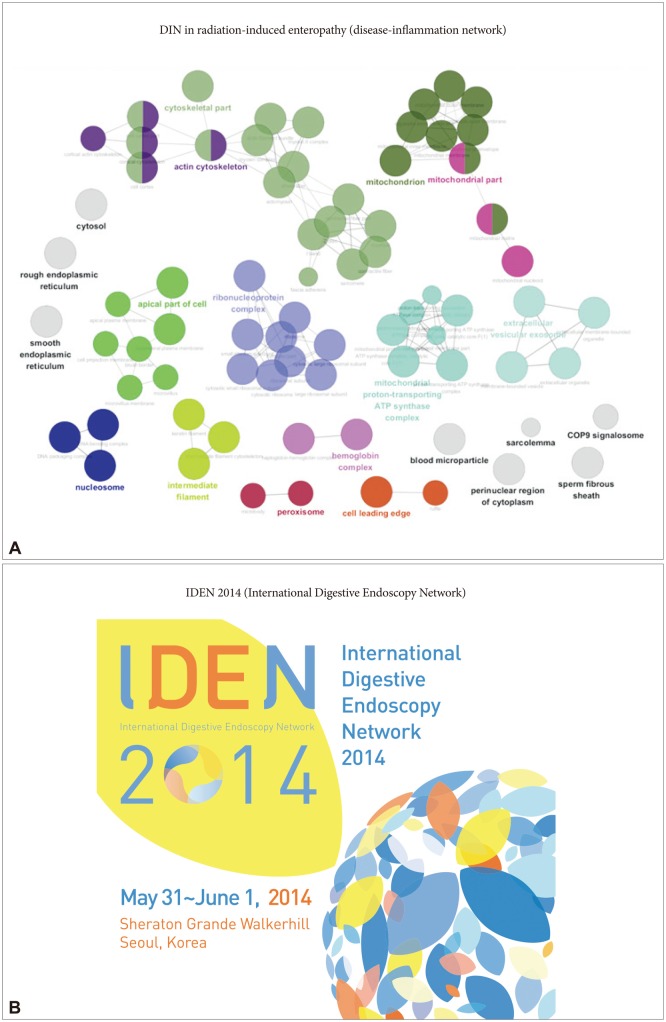
Fig.┬Ā2(A, B) Asian Young Endoscopist Award (AYEA) forum. The AYEA forum was excellently organized by Professor Jong Ho Moon (circled, Soonchunhyang University Bucheon Hospital, Director of Liaison Committee, The Korean Society of Gastrointestinal Endoscopy) and successfully executed during International Digestive Endoscopy Network 2014. 
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
