AbstractGastric cancer screening is common in countries with high prevalence rates of gastric cancer. However, data supporting the effectiveness of gastric cancer screening are lacking. Thus, the aim of this review was to examine the current evidence on gastric cancer screening. Herein, we reviewed radiographic and endoscopic tests as methods of gastric cancer screening. Previous cohort studies and case-control studies have demonstrated reduced gastric cancer mortality in study populations that had undergone gastric cancer screening with radiographic tests. Recently, a case-control study in Japan reported a 30% reduction in gastric cancer mortality when screening was undertaken via endoscopy. Also, endoscopic screening for gastric cancer exhibited higher sensitivity and specificity than radiographic screening. Moreover, most cost-effectiveness analyses on the best strategy for detecting early gastric cancer have generally concluded that endoscopy is more cost-effective than radiographic testing. Although data on the impact of endoscopy screening programs on gastric cancer mortality are limited, recent study results suggest that gastric cancer screening by endoscopy in average-risk populations performs better than radiography screening. Further evaluation of the impact of these screening methods should take into account cost and any associated reduction in gastric cancer mortality.
INTRODUCTIONUntil recently, gastric cancer comprised the second most common type of cancer worldwide. In 2012, gastric cancer fell to fifth place, behind lung, breast, colorectum, and prostate cancers.1 Nevertheless, gastric cancer remains the third most common cause of cancer death.1 Asian countries, including China, Japan, and Korea, in particular, report some of the highest incidences of gastric cancer in the world.2 While its incidence has declined in Korea in recent decades, gastric cancer was still the second most common cancer in the country in 2010 (crude incidence rate: 60.3 per 100,000; age-standardized incidence rate: 41.8 per 100,000).3
In countries with high prevalence rates of gastric cancer, like China, Japan, and Korea, gastric cancer screening is common. Since 1960, as a result of mass gastric cancer screening implementation using photofluorography (via indirect upper gastrointestinal series [UGIS]), which facilitates early detection, Japan has achieved improvements in survival and cure rates.4,5,6 In recent years, endoscopy has been increasingly utilized in opportunistic screening for gastric cancer.7 In Korea, the nationwide gastric cancer screening program has provided endoscopy and UGIS as initial screening methods.8 However, apart from the countries where gastric cancer is highly prevalent, many countries generally lack national guidelines or recommendations for gastric cancer screening. Additionally, data in support of the effectiveness of gastric cancer screening are lacking.
Thus, the aim of this review was to examine current evidence on gastric cancer screening and to determine the most cost-effective screening strategies. Herein, we reviewed radiographic and endoscopic tests as screening methods for gastric cancer, as these two methods are most commonly provided by population-based organized screening programs.
CURRENT POPULATION-BASED GASTRIC CANCER SCREENING PROGRAMSThe incidence and mortality of gastric cancer differ significantly by region, population, and race distribution. Additionally, healthcare systems, health resources, and social and economic conditions may greatly affect gastric cancer prevention and screening strategies. At present, the East Asia region, particularly Japan, Korea, and other countries with high incidence rates of gastric cancer, have achieved tangible results from their screening programs, as well as from preventive interventions. However, no nationwide screening of gastric cancer has been reported in the United States, Europe, or other areas with low incidence rates of gastric cancer.
JapanAround 1960, gastric cancer screening via photofluorography began to be offered in the Miyagi prefecture and has since been adopted nationwide.9 In 1983, under the Health Service Law for the Aged, annual gastric cancer screening via photofluorography was introduced for all residents aged 40 years and older. The screening rate for gastric cancer was 11.8% in 2007, following a trend of declining participation in screening programs since the early 1990s.10 In 2008, a Japanese research group for cancer screening recommended guidelines for gastric cancer screening.9 To do so, they evaluated four gastric cancer screening methods: photofluorography, endoscopy, serum pepsinogen testing, and Helicobacter pylori antibody testing. On the basis of a benefit/harm balance, the research group recommended gastric cancer screening using photofluorography for both population-based and opportunistic screening.9 The other methods were not recommended for population-based screening because of insufficient evidence.9 Notwithstanding, endoscopy has recently replaced photofluorography as the initial mass screening method in several Japanese cities.11
KoreaIn Korea, the Korean Gastric Cancer Association and National Cancer Center established national guidelines for gastric cancer screening in 2001. These guidelines recommend biennial gastric cancer screening for men and women aged 40 years or older via either UGIS or endoscopy.12 Based on these guidelines, a nationwide gastric cancer screening program was started in 2002 as part of the National Cancer Screening Program (NCSP). The NCSP now provides support for biennial gastric cancer screening via either UGIS or endoscopy for Medical Aid Program recipients and National Health Insurance beneficiaries aged 40 or older.13 The participation rate for gastric cancer screening provided by the NCSP increased from 12.7% in 2002 to 43.9% in 2012.14,15 In addition to the NCSP, opportunistic gastric cancer screening is also widely available in Korea. According to the Korean National Cancer Screening Survey, the participation rate for opportunistic and organized gastric cancer screening has increased significantly, from 39.2% in 2004 to 70.9% in 2012.16
Other countries in AsiaIn China, although gastric cancer is the second most common cancer, no nationwide screening program has been established. Therefore, early detection of gastric cancer relies on opportunistic screening alone, although endoscopy is widely available in major cities. Notwithstanding, UGIS and serum pepsinogen testing are not commonly practiced in China for reasons of cost and availability.7
Singapore also lacks a nationwide gastric cancer screening program for the population.7 As the risk of developing gastric cancer in Singapore is deemed intermediate, screening is more effectively targeted at high-risk groups than at the population level. A cost-benefit analysis of gastric cancer screening conducted in Singapore showed that screening by endoscopy was most cost-effective in moderate- to high-risk populations.17
In Taiwan, gastric cancer screening is limited to the high-risk population. Two prevention campaigns have been implemented in Matzu Island where the prevalence and incidence of gastric cancer were exceptionally high.18,19 From 1995 to 2004, subjects with low serum concentrations on the serum pepsinogen test (<30 ng/mL) received endoscopy.18 From 2004 to 2005, those with H. pylori infection underwent endoscopy and H. pylori eradication,19 and this population-based program showed that H. pylori eradication significantly reduced the incidence of atrophic gastritis and gastric cancer.20
EVIDENCE ON GASTRIC CANCER SCREENINGThe efficacy of cancer screening is best assessed with randomized controlled trials to determine mortality reductions in screened versus unscreened subjects. Although randomized controlled trials represent the most reliable method for evaluating the impact of screening on cancer risk, such intervention studies are not feasible in most countries because gastric screening is already widely conducted. Furthermore, obtaining accurate estimates of mortality reductions requires long-term follow-up of large populations.
Radiographic tests (photofluorography, UGIS, or barium-meal studies)No randomized controlled trial addressing the use of radiographic tests in gastric cancer screening has been published. Meanwhile, four case-control studies and four cohort studies have been conducted to evaluate the effect of gastric cancer screening by photofluorography on mortality (Table 1); one case-control study was conducted in Venezuela,21 while the others were conducted in Japan.4,22,23 Most of the case-control studies conducted in Japan demonstrated a 40% to 60% reduction in gastric cancer mortality with photofluorography screening.4,23,24 Nonetheless, the study conducted in Venezuela reported no detectable reduction in gastric cancer mortality. Fukao et al.,4 Oshima et al.,22 and Abe et al.23 reported that the summary odds ratios of three case-control studies for ever-screened versus never-screened subjects were 0.39 (95% confidence interval [CI], 0.29 to 0.52) for men and 0.50 (95% CI, 0.34 to 0.72) for women in reducing gastric cancer mortality.24 They discerned that the Japanese screening program with photofluorography is effective in reducing gastric cancer mortality.
Regarding the four cohort studies, one study lacked statistical power and did not show a significant difference in the relative risk (RR) of mortality.25 The cohort study conducted by Mizoue et al.5 revealed significantly reduced mortality in men (RR, 0.54; 95% CI, 0.41 to 0.70), while the reduction in mortality was not significant in women (RR, 0.74; 95% CI, 0.51 to 1.07). Another cohort study with a 13-year follow-up in Japan reported a 40% reduction in gastric cancer mortality in screened versus unscreened subjects (RR, 0.60; 95% CI, 0.43 to 0.83).26 The most recent study, conducted by Miyamoto et al.,27 also reported a significant mortality reduction in both men and women who had undergone screening versus those who had not (RR, 0.52; 95% CI, 0.36 to 0.75 in men; RR, 0.51; 95% CI, 0.27 to 0.96 in women).
EndoscopyOnly a few studies have evaluated the effectiveness of endoscopic screening in terms of mortality reduction (Table 2). One community-based case-control study was conducted to evaluate the reduction in gastric cancer mortality by endoscopic screening.28 In this study, compared to patients who had never undergone screening for gastric cancer, the odds ratios were 0.695 (95% CI, 0.489 to 0.986) for patients who underwent endoscopic screening and 0.865 (95% CI, 0.631 to 1.185) for those who underwent radiographic screening.
Regarding cohort studies, one study was conducted in an area with a high incidence of gastric cancer in Linqu County, China.29 From 1989 to 1999, endoscopic screening was conducted for 4,394 residents. Both the incidence and mortality rates of gastric cancer were monitored until 2000. Over this period, 85 cases of gastric cancers were detected, 29 of which were early cancers. However, compared with the overall mortality for Linqu County, the standard morality ratio was 1.01 (95% CI, 0.72 to 1.37). Another cohort study conducted in Japan by Hosokawa et al.30 reported that the RR for gastric cancer death in the examined group was 0.347 (95% CI, 0.140 to 0.860) when compared with the nonexamined group. The RRs in men and women were 0.217 (95% CI, 0.068 to 0.699) and 0.684 (95% CI, 0.160 to 2.929), respectively. In conclusion, they recommended the application of endoscopy in population-based screening programs for gastric cancer in regions or countries where gastric cancer mortality is high.
TEST ACCURACYUsually, the accuracy of a screening test is indicated by its sensitivity and specificity. A good screening test must have high sensitivity and high specificity. The most common method used to calculate sensitivity and specificity involves the follow-up of persons who have undergone screening to ascertain the number of cancer cases occurring among them via record linkage to a population-based cancer registry. Table 3 summarizes the reported sensitivities and specificities of radiographic and endoscopic tests as screening methods.
The sensitivity of photofluorography reported from the Japanese studies ranged from 56.8% to 88.5%, whereas specificity ranged from 81.3% to 92.0%.31,32,33,34,35,36 Two studies related to the use of endoscopy as a diagnostic test have reported on the accuracy of endoscopy.37,38 In the first study, the sensitivity of endoscopy was found to be 77.8%, based on a 3-year follow-up using the cancer registry system of the Fukui prefecture.37 The other study based on a follow-up survey of individual participants recorded an 84.0% sensitivity for endoscopy.38
Two recent studies have compared the accuracies of radiographic tests and endoscopy.39,40 One study conducted in Korea utilized data obtained from a population-based national cancer screening database. In that study, the sensitivities of UGIS and endoscopy screening to detect gastric cancer were 36.7% and 69.0%, with specificities of 96.1% and 96.0%, respectively. The other study conducted in Japan reported sensitivities of prevalence screening calculated by the incidence method of 89% for endoscopic screening and 83% for radiographic screening; however, the difference was not significant. The specificities of endoscopy and radiographic screening were 85% and 86%, respectively. The differences in the sensitivities and specificities between Korea and Japan might be affected by the differences in their respective screening intervals: every 2 years in Korea and every year in Japan. Also, the quality of cancer registry data in the Japanese study was not good, as some of the interval cancers were lost because of insufficient follow-up, and the sensitivity might have been overestimated. Nevertheless, both studies showed that the sensitivity of endoscopic screening for gastric cancer was higher than that of radiographic screening and suggested there should be greater use of endoscopy in gastric cancer screening.
COST-EFFECTIVENSS AND PREFERENCEIn the United States, where gastric cancer incidence rates are low, one study comparing endoscopy versus no screening concluded that one-time screening for the general population at the age of 50 would cost US $115,664 per quality-adjusted life year (QALY).41 Another study in the United States concluded that endoscopy screening of less advanced lesions was not cost-effective, except possibly for immigrants from high-risk Asian countries.42
In many Asian countries experiencing high gastric cancer burden, cost-effectiveness analyses of gastric cancer screening are ongoing. In Singapore, where the risk of gastric cancer is low to intermediate, annual endoscopy was deemed the optimal strategy, with an incremental cost-effectiveness ratio (ICER) of US $44,098 per QALY compared to no screening, while biennial endoscopy was estimated as the most cost-effective strategy with an ICER of US $25,949 per QALY.43 The ICERs per QALY in the Singaporean study were lower than those in the United States because of differences in gastric cancer prevalence, healthcare resources, screening strategy costs, and other healthcare-related factors. A study conducted in Taiwan reported that annual screening using endoscopy for high-risk populations whose pepsinogen-I levels were <30 ng/mL at the age of 50 years versus no screening resulted in an ICER of US $29,741 per life year gained.44 In a Japanese study, endoscopy was deemed the best method for detecting early gastric cancer and the cheapest strategy with regard to the cost of identifying one case of gastric cancer.11
The Korean government provides gastric cancer screening as a part of the NCSP and requires many studies to report on the cost-effectiveness thereof. Lee at al.45 reported that endoscopy was more cost-effective than UGIS until the cost per cancer case detected becomes 3.7-fold more expensive than UGIS. Another study by Chang et al.46 using a time-dependent Markov model to compare 13 different screening alternatives reported annual endoscopy from ages 50 to 80 years was the most cost-effective strategy in men, and biennial endoscopy from ages 50 to 80 years was the most cost-effective in women. Cho et al.47 reported that endoscopy ICERs (119,099,000 to 178,700,000 Korean won/survival) were lower than upper gastrointestinal X-ray ICERs (260,201,000 to 371,011,000 Korean won/survival). In Korea, three studies comparing endoscopy versus no screening agreed on endoscopy as the more cost-effective strategy.
In financial terms, the test is not as cost-effective if the cost is too high. The cost of endoscopy is reportedly 3- to 4-fold more expensive than that of radiography testing (photofluorography) in Japan.11,48 However, in Korea, the cost of endoscopy is about the same as that of radiographic testing (UGIS) (unit costs for endoscopy and UGIS were US $34.89 and US $32.67, respectively, in 2008).45
For a successful screening program, a high level of participation is required, which likely depends on individual attitudes and preferences about the screening method used. In Korea, endoscopy is the preferred method for gastric cancer screening. According to a Korean National Cancer Screening Survey in 2006, 67% of individuals chose endoscopy as their preferred gastric cancer screening method, while 33% chose UGIS as their preferred method.49 Furthermore, the proportion of people choosing endoscopy screening provided by the NCSP has continued to increase annually, from 24.8% in 2002 to 70.8% in 2011 (Fig. 1).50
CONCLUSIONSEndoscopy is widely conducted in clinical settings as a part of routine health check-ups. Some authors have reported higher detection rates of early-stage gastric cancer with endoscopy compared to radiographic tests and have thus concluded that endoscopy is a more sensitive screening method.11,39,40,51 Despite the diagnostic advantages of endoscopy, data on the impact of endoscopy screening programs on gastric cancer mortality are limited.
Notwithstanding, the results of recent studies suggest that application of endoscopy in gastric cancer screening programs is more cost-effective than screening by radiographic tests in average-risk populations. Also, the general population is more likely to prefer gastric cancer screening by endoscopy. Nonetheless, further study of the impact of endoscopy on gastric cancer mortality is needed, and future evaluations of screening methods should take into account cost and any associated reduction in gastric cancer mortality.
References1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer; c2014. cited 2014 Apr 14]. Available from: http://globocan.iarc.fr.
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69ā90. 21296855.
3. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1ā14. 23613665.
4. Fukao A, Tsubono Y, Tsuji I, S HI, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer 1995;60:45ā48. 7814150.
5. Mizoue T, Yoshimura T, Tokui N, et al. Prospective study of screening for stomach cancer in Japan. Int J Cancer 2003;106:103ā107. 12794764.
6. Hisamichi S, Sugawara N, Fukao A. Effectiveness of gastric mass screening in Japan. Cancer Detect Prev 1988;11:323ā329. 3390854.
7. Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008;9:279ā287. 18308253.
8. Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol 2008;38:327ā333. 18407932.
9. Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2008;38:259ā267. 18344316.
10. Yoshida M, Kondo K, Tada T. The relation between the cancer screening rate and the cancer mortality rate in Japan. J Med Invest 2010;57:251ā259. 20847525.
11. Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 2006;12:4873ā4874. 16937471.
12. Park CH. Proposal of a screening program for gastric cancer in Korea. J Korean Med Assoc 2002;45:964ā971.
13. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725ā730. 21627372.
14. Lee YY, Oh DK, Choi KS, Jung KW, Lee HY, Jun JK. The current status of gastric cancer screening in Korea: report on the National Cancer Screening Programme, 2009. Asian Pac J Cancer Prev 2011;12:3495ā3500. 22471504.
15. National Cancer Center. Cancer Facts & Figures 2014. Goyang: National Canceer Center; 2014. p. 124.
16. Suh M, Choi KS, Lee YY, Jun JK. Trends in cancer screening rates among Korean men and women: results from the Korean National Cancer Screening Survey, 2004-2012. Cancer Res Treat 2013;45:86ā94. 23864841.
17. Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol 2006;4:709ā716. 16765306.
18. Liu CY, Wu CY, Lin JT, Lee YC, Yen AM, Chen TH. Multistate and multifactorial progression of gastric cancer: results from community-based mass screening for gastric cancer. J Med Screen 2006;13(Suppl 1):S2āS5. 17227633.
19. Lee YC, Wu HM, Chen TH, et al. A community-based study of Helicobacter pylori therapy using the strategy of test, treat, retest, and re-treat initial treatment failures. Helicobacter 2006;11:418ā424. 16961802.
20. Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676ā682. 22698649.
21. Pisani P, Oliver WE, Parkin DM, Alvarez N, Vivas J. Case-control study of gastric cancer screening in Venezuela. Br J Cancer 1994;69:1102ā1105. 8198977.
22. Oshima A, Hirata N, Ubukata T, Umeda K, Fujimoto I. Evaluation of a mass screening program for stomach cancer with a case-control study design. Int J Cancer 1986;38:829ā833. 3793262.
23. Abe Y, Mitsushima T, Nagatani K, Ikuma H, Minamihara Y. Epidemiological evaluation of the protective effect for dying of stomach cancer by screening programme for stomach cancer with applying a method of case-control study: a study of a efficient screening programme for stomach cancer. Nihon Shokakibyo Gakkai Zasshi 1995;92:836ā845. 7783375.
24. Tsubono Y, Hisamichi S. Case-control studies of screening for gastric cancer in Japan. J Gastroenterol Mass Surv 1999;37:182ā185.
25. Inaba S, Hirayama H, Nagata C, et al. Evaluation of a screening program on reduction of gastric cancer mortality in Japan: preliminary results from a cohort study. Prev Med 1999;29:102ā106. 10446035.
26. Lee KJ, Inoue M, Otani T, et al. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer 2006;118:2315ā2321. 16331632.
27. Miyamoto A, Kuriyama S, Nishino Y, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med 2007;44:12ā19. 16956654.
28. Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One 2013;8:e79088. 24236091.
29. Riecken B, Pfeiffer R, Ma JL, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med 2002;34:22ā28. 11749093.
30. Hosokawa O, Miyanaga T, Kaizaki Y, et al. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol 2008;43:1112ā1115. 18609154.
31. Suguhara N, Shibuki S, Hirasawa Y, Morimoto T. Characteristics of false negative cases in gastric cancer mass survey. Stomach Intest 1991;26:1357ā1362.
32. Fukao A, Hisamichi S, Takano A, Sugahara N. Accuracies of mass screening for gastric cancer: test sensitivity and program sensitivity. J Gastroenterol Mass Surv 1992;97:59ā63.
33. Ishida T, Suematsu T, Oobayashi K, Takada Y, Kimura S, Suematsu C. Measurement of accuracy of stomach mass screening by population-based cancer registration. J Gastroenterol Mass Surv 1994;32:9ā16.
34. Hosokawa O. Transition in the diagnostic methods for gastric cancer and screening for gastric cancer. J Gastroenterol Mass Surv 1995;33:195ā198.
35. Abe S, Shibuya D, Noguchi T, Shimada T. An estimate of the false-negative rate of mass-screening for gastric carcinoma. J Gastroenterol Mass Surv 2000;38:475ā482.
36. Murakami R, Tsukuma H, Ubukata T, et al. Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer 1990;65:1255ā1260. 2302674.
37. Hosokawa O, Hattori M, Takeda T, Watanabe K, Fujita M. Accuracy of endoscopy in detecting gastric cancer. J Gastroenterol Mass Surv 2004;42:33ā39.
38. Otsuji M, Kouno Y, Otsuji A, Tokushige J, Shimotatara K, Arimura K. Assessment of small diameter panendoscopy for diagnosis of gastric cancer: comparative study with follow-up survey date. Stomach Intest 1989;24:1291ā1297.
39. Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 2012;7:e50041. 23209638.
40. Hamashima C, Okamoto M, Shabana M, Osaki Y, Kishimoto T. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013;133:653ā659. 23364866.
41. Gupta N, Bansal A, Wani SB, Gaddam S, Rastogi A, Sharma P. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc 2011;74:610ā624. 21741639.
42. Yeh JM, Hur C, Kuntz KM, Ezzati M, Goldie SJ. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer 2010;116:2941ā2953. 20564399.
43. Zhou HJ, Dan YY, Naidoo N, Li SC, Yeoh KG. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS One 2013;8:e83959. 24386314.
44. Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev 2007;16:875ā885. 17507609.
45. Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol 2010;16:245ā250. 20066745.
46. Chang HS, Park EC, Chung W, et al. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in South Korea: a cost-utility analysis. Asian Pac J Cancer Prev 2012;13:2721ā2728. 22938448.
47. Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013;14:2533ā2540. 23725170.
48. Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol 2007;13:4316ā4320. 17708603.
49. Choi KS, Kwak MS, Lee HY, Jun JK, Hahm MI, Park EC. Screening for gastric cancer in Korea: population-based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol Biomarkers Prev 2009;18:1390ā1398. 19383892.
50. National Cancer Center. Support and Evaluation of National Cancer Control Program Report. Goyag: National Cancer Center; 2014.
Fig.Ā 1Trends in the use of upper gastrointestinal (UGI) series and endoscopy among participants in the National Cancer Screening Program in Korea from 2002 to 2011. 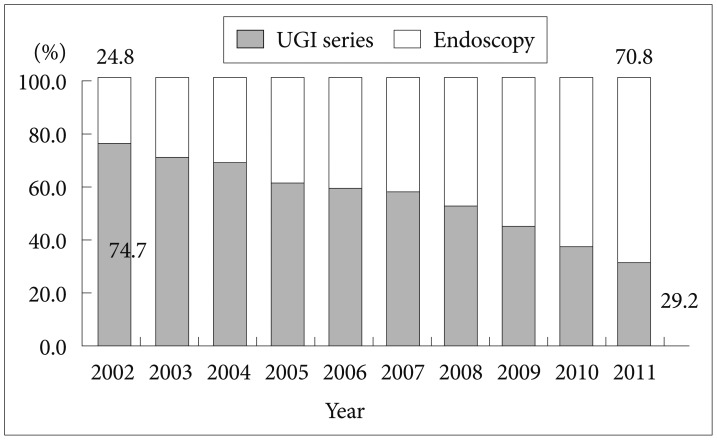
TableĀ 1Observational Studies of Gastric Cancer Screening by Upper Gastrointestinal Series 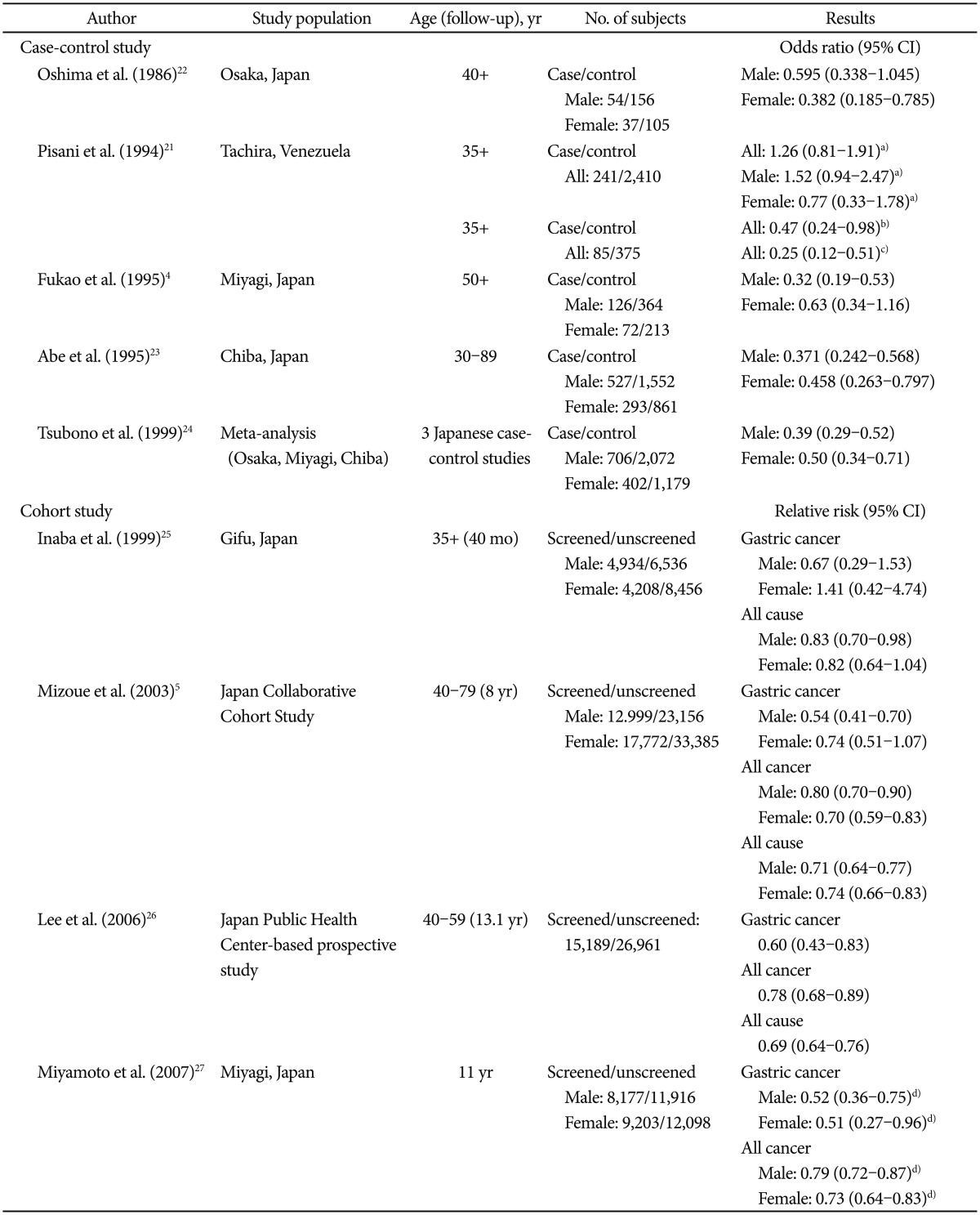 CI, confidence interval. a)Excluded cases within 6 months before the date of diagnosis; b)Excluded cases within 1 month before the date of diagnosis among subjects who underwent at least one screening test; c)Excluded cases within 6 months before the date of diagnosis among subjects who underwent at least one screening test; d)Adjusted for age or age and sex. |
|
|||||||||||||||||||||||||||||||||||||||||||||||
