AbstractBackground/AimsAlthough the advantages of endoscopic submucosal dissection (ESD) are well established, there are important limitations that relate to its higher cost and higher rate of complications compared with endoscopic mucosal resection. This study assessed the therapeutic safety and efficacy of ESD in the treatment of small gastric dysplasia and early gastric cancer (EGC) located within the antrum in an outpatient setting, and it compared the results with those from patients admitted to hospital for ESD treatment.
MethodsThis study was a retrospective analysis of a prospectively maintained database. We reviewed consecutive patients with EGC or gastric dysplasia who underwent ESD between October 2007 and May 2008. The lesions were smaller than 2 cm and were located in the antrum. We analyzed 105 lesions in 105 patients. The patients were assigned to two groups according to each patient's preference.
ResultsThe overall rates of complete resection were 98.1% in the inpatients group and 94.3% in the outpatients group. Immediate bleeding occurred in four inpatients, which included one patient in the outpatient group. Delayed bleeding occurred in one inpatient within 24 hours of the procedure. Macroperforations did not occur in either group. A microperforation was found in one outpatient.
INTRODUCTIONEndoscopic submucosal dissection (ESD) was developed to enable safe en bloc resections of lesions that are larger than 2 cm and of lesions with ulceration.1 This procedure can be used to resect lesions with distinct margins for pathological evaluation, regardless of their size.2 Therefore, ESD is considered an effective therapy for the treatment of early gastric neoplasms.1,3 However, compared with endoscopic mucosal resection (EMR), ESD has several limitations that include longer procedural completion times, the requirement for the operator to have advanced skills, and higher risks of hemorrhage and perforation.4
Recent studies have demonstrated an acceptable rate of complications, all of which can be adequately managed endoscopically.1,5 In addition, the studies showed that the en bloc resection rate for lesions located within the antrum was significantly higher than that for other lesions and that the occurrence of delayed bleeding was independently higher in large lesions than in small lesions (<20 mm).5
Another important limitation is that ESD is more expensive than EMR, with the costs of ESD varying according to the hospital and country in which it is performed. However, it is almost certain that ESD for inpatients costs more than that for outpatients. If ESD can be performed on small antral lesions in an outpatient setting, it would be more cost-effective. Although investigations into the safety and efficacy of ESD have been conducted, no studies have investigated its usefulness in the treatment of day patients with gastric dysplasia or early cancer.
Therefore, this study assessed the therapeutic safety and efficacy of ESD in the treatment of small gastric dysplasia and early gastric cancer (EGC) located within the antrum in an outpatient setting, and it compared the results with those from patients admitted to the hospital.
MATERIALS AND METHODSPatientsAll patients who were diagnosed with gastric dysplasia or EGC within the antrum, had lesions that were smaller than 2 cm, and were treated using ESD as inpatients or outpatients from October 2007 to May 2008 at Kyungpook National University Hospital, Daegu, Korea, were enrolled to participate in this study. Patients were assigned to two groups based on each patient's preference. We retrospectively reviewed and analyzed 105 lesions from 105 patients within the ESD database. The hospital's institutional review board approved this study.
MethodsTreatment flowsWhen a gastric adenocarcinoma was diagnosed prior to ESD, metastases were ruled out using other diagnostic modalities, including endoscopic ultrasound, abdominal computed tomography, and/or positron emission tomography. Inpatients were admitted one day before they underwent ESD. ESD was performed by a single endoscopist (SWJ) who had performed more than 500 gastric ESD procedures. After the resection of the lesion, we performed electrocauterization on the visible vessels to prevent bleeding. Both groups of patients had their vital signs checked and they were assessed for the presence of pain in the recovery room immediately after ESD. The inpatients returned to the ward and the outpatients remained in the recovery room that is located in the endoscopy center, for 1 to 2 hours. We routinely checked simple chest radiographs in both groups. The doctors then allowed the outpatients to go home. Patients in both groups were assigned nothing per oral statuses for 4 hours, then they were allowed clear water for the next 12 hours and soft diets on the second day. The inpatients were discharged the next day and the outpatients were discharged once they had fully recovered. A proton pump inhibitor (PPI), pantoprazole (40 mg), was administered intravenously on the day of the procedure, and then it was administered orally for 2 months from the second day after the ESD procedure in the case of the inpatients, and orally from the first day after the ESD procedure in the case of the outpatients. Second-look endoscopies were not performed in either group.
Endoscopic characteristics of the lesionsThe endoscopy reports provided information about the locations of the tumors (anterior wall, posterior wall, greater curvature, or lesser curvature of stomach), the sizes of the lesions in relation to the lengths of the long diameters, the gross appearances of the lesions (elevated, flat, or depressed), and the presence of ulceration.
The gross appearances of the tumors were categorized according to the Japanese classification for EGC.6 Type I, type IIa, type I+IIa, and type IIa+IIc; a combination of these two types was categorized as elevated, type IIc, type III, and type IIc+IIa; a combination of these two types was categorized as depressed, and type IIb was categorized as flat. An ulcer was defined as a lesion with a converging fold, mucosal deformity, and/or mucosal defects on endoscopic examination.
Characteristics of the resected lesionsAn en bloc resection was defined as a resection in one piece as opposed to a piecemeal resection. The complete resection was defined as en bloc lesion resection or piecemeal resection that can complete reconstitution of the lesion by tissues with negative lateral and vertical resection margin and no lymphovascular involvement. Resection specimens were stretched with needles and stents for histopathological assessments, and they were sectioned perpendicularly at 2 mm intervals. The histological specimens were categorized into adenocarcinomas, which included well or moderately differentiated papillary adenocarcinomas or signet ring cell carcinomas, and dysplasia, which included mild and severe dysplasia.
Evaluation of complications associated with gastric ESDThe complications evaluated included bleeding and perforations after ESD.4,7 Bleeding was categorized as immediate or delayed. Immediate bleeding was defined as the occurrence of a hemorrhage during ESD, and was categorized into four grades: (1) grade 0 that was defined as no hemorrhages during the procedure; (2) grade 1 that was defined as a very small amount of bleeding that stopped spontaneously or easily after a single hemocoagulation procedure; (3) grade 2 that was defined as a small amount of bleeding that was easily stopped with multiple hemocoagulation procedures or hemoclips; and (4) grade 3 that was defined as a large amount of bleeding that required multiple hemocoagulation procedures and hemoclips to stop it. The patients were divided into the non-bleeding group (grades 0 and 1) and the bleeding group (grades 2 and 3). Delayed bleeding was defined as a hemorrhage after ESD, and included one of the following: hematemesis or melena, unstable vital signs, or a reduction in the hemoglobin level of more than 2 g/dL after ESD.7
Perforations were divided into macroperforations and microperforations. A macroperforation was defined as a perforation that was diagnosed using endoscopy during or immediately after ESD, and a microperforation was defined as the identification of free air only on a simple chest radiograph after ESD.
Statistical analysisThe data were analyzed using IBM SPSS version 18.0 (IBM Co., Armonk, NY, USA). We analyzed the differences between the inpatient and outpatient groups in relation to the patients' clinical characteristics, the characteristics of the lesions in relation to their sizes, appearances, and pathological features, the numbers of en bloc resections, the numbers of complete resections, and the frequencies of complications, including microperforations, macroperforations, immediate bleeding, and delayed bleeding. The differences between the groups were evaluated using the t-test for continuous data, and the chi-square test or Fisher exact test for categorical variables. p-values of <0.05 were considered to indicate statistical significance.
RESULTSBaseline clinicopathological characteristicsFifty-two patients underwent ESD as inpatients and 53 patients underwent ESD as outpatients. The mean┬▒SD ages of the patients were 60.9┬▒10.1 years (range, 36 to 79) in the inpatient group and 59.1┬▒8.5 years (range, 40 to 76) in the outpatient group. The ratios of males to females were 34:18 in the inpatient group and 35:18 in the outpatient group.
Patients with one or more underlying conditions accounted for 36.5% (19/52) of the inpatient group and 28.3% (15/53) of the outpatient group (p=0.681). Five patients in the inpatient group and two patients in the outpatient group took aspirin or other antiplatelet agents for cardiovascular or other diseases (p=0.282) (Table 1).
The mean┬▒SD total diameter of the resected lesions was 32.5┬▒7.9 mm (range, 13 to 55) in the inpatient group and 31.4┬▒6.2 mm (range, 18 to 55) in the outpatient group (p=0.299). Ulcers were observed in 28 patients, and 16 patients in the inpatient group had ulcers and 12 patients in the outpatient group had ulcers (p=0.346).
Dysplasia was evident in 26 lesions (50.0%) in the inpatient group and 32 lesions (60.4%) in the outpatient group. EGC was identified in 26 lesions (50.0%) in the inpatient group and 21 lesions (39.6%) in the outpatient group (p=0.285).
The gross appearance of the lesions indicated that the elevated type was more common than the flat or depressed types in both groups; however, this difference was not statistically significant. The characteristics of the lesions are shown in Table 2.
Results of ESDRates of en bloc resection and complete resectionThe rate of en bloc resection was 99.0% (104/105) for all lesions, and it was 98.1% (51/52) in the inpatient group and 100% (53/53) in the outpatient group (p=0.310). A complete resection was achieved for 96.1% (101/105) of all lesions, and complete resection was achieved for 98.1% (51/52) of the lesions in the inpatient group and for 94.3% (50/53) of the lesions in the outpatient group (p=0.346) (Table 3).
Development of complicationsPerforations or bleeding were observed in 6.7% (7/105) of all patients who underwent ESD, and they were observed in 9.6% (5/52) of the inpatients and in 1.9% (2/53) of the outpatients. There was no difference between the groups in relation to the occurrence of complications (p=0.230) (Table 3). Four patients in the inpatient group and one patient in the outpatient group had immediate bleeding that was higher than grade 2; however, none of the patients required blood transfusions. Delayed bleeding occurred in one patient in the inpatient group within 24 hours of the procedure; he recovered with endoscopic intervention and conservative treatment, and he did not require surgical intervention. Macroperforations did not occur in either of the groups. A microperforation occurred in one patient in the outpatient group who recovered after conservative treatment that included no oral intakes, antibiotics, fluid support, and pain control after admission.
Cases requiring hospital admission in the outpatient groupFour patients in the outpatient group were hospitalized after ESD. Table 4 summarizes the clinicopathological features of these four cases. Four patients developed abdominal pain and they were hospitalized after the procedure to control the pain. In case 4, the patient was hospitalized in order to control microperforation. In all four patients, there was no occurrence of further complications related to ESD, and they recovered with conservative treatment.
DISCUSSIONESD can treat lesions of different sizes and shapes, and several reports have demonstrated the safety and effectiveness of ESD.1,8,9 Although ESD is a less aggressive procedure compared with surgery, it is associated with a higher risk of complications than conventional EMR. However, the development of devices and the refinement of techniques over several years have reduced complications rates.1,5
The major complications associated with ESD have been reported as bleeding, perforations, and pain.2,4,10 Of these, bleeding has been reported as the most common complication.7 The reported frequency of bleeding complications varies between 1.5% and 24%, with the variation being a consequence of the definitions used and the types of lesions resected.2,11 In the present study of 105 cases, bleeding complications occurred in six cases (5.7%), and most were minor bleeding complications that occurred during or after the procedure without any accompanying changes in the vital signs. The bleeding was stopped using endoscopic (hemocoagulation or hemoclipping) and conservative treatments, and neither surgery nor transfusions were required. Immediate bleeding developed in five out of the 105 cases (4.8%) in our study. For the endoscopist, delayed bleeding that occurs after discharge is the most worrying event. Delayed bleeding developed in only one case (0.9%). Several studies have reported risk factors that are associated with post-ESD bleeding, and the results of these studies are diverse.12 However, a higher occurrence of delayed bleeding is reportedly independently associated with larger lesions, the location of the lesions in the upper regions of the body, the presence of ulceration or scars, and prolonged procedure times.2,5,13 These results support the treatment of antral and small lesions within outpatient clinics. The lack of consistent results requires further consideration.
Perforation is another major complication that is associated with ESD, the frequency of which has been reported as between 0% and 6.7%.2,14,15 Although the treatment of perforations has not been standardized based on the size or the degree of complications, such as peritonitis, in our study, only one (0.9%) perforation occurred, which was managed with conservative treatment without the need for further surgery. Large tumors, the location of the lesions in the upper regions of the body, and long procedure times are known risk factors for perforation following ESD.16 Therefore, ESD for the treatment of small antral lesions was safe in relation to bleeding and perforation.
Localized pain that was just above the resected lesion tended to develop after the procedure, and it was controlled by a PPI, which was administered once a day at a standard dose, or analgesics.17 However, physicians do not tend to have much interest in controlling epigastric pain after ESD. A recent report that described pain control using fentanyl patches after ESD emphasized the importance of pain control.18 In our study, four patients in the outpatient group were hospitalized because of epigastric pain. All patients showed improvements when they were treated conservatively, which involved no oral intakes, fluid therapy, the administration of analgesics, and PPI medication, and they were discharged from hospital. Abdominal pain, tenderness, or rigidity involving the entire abdomen developed in one patient (case 4) with microperforation. This patient was admitted to the hospital for observation and improved with supportive care. Pain impacts upon patients' health, the quality of patients' lives, the length of hospital stays, and healthcare costs. Increasingly, many physicians are interested in achieving successful results and successfully managing pain after operations and procedures. The useful preventive strategy for control of pain should be validated in future studies.
In the current study, incompletely resected lesions were identified in four out of 105 lesions, resulting in a complete resection rate of 96.1%. No significant difference was found between the inpatient and the outpatient groups with respect to the complete resection rate. This short-term oncologic outcome also supports the usefulness of ESD of antral lesions in a day-patient setting.
Hospital admission days before and after gastric ESD are different according to the strategies of each center, averaging more than 3 days in clinical practice, which leads to elevation of medical and social costs for patients care. The results of this study showed that ESD of small gastric antral lesions within an outpatient setting has similar outcomes in relation to complete resection rates and complication rates. Antral lesions are easy to access using endoscopy, and they are the best candidate lesions for novice endoscopists who are eager to learn and to perform gastric ESD, because most gastric tumors are located in the antrum and resection of these lesions using ESD is relatively easy. Some reports have demonstrated a learning curve for ESD of the antrum, which was 90% of lesions treated by beginners with experience of fewer than 30 cases.19,20 The findings from this study may contribute to future assessments of the ways in which patients with small antral gastric tumors are treated, which may involve office-based EMR or ESD.
This study has several limitations, the first of which relates to a possible selection bias. Although the data were prospectively collected based on each patient's preferences, this was not a randomized study. However, the ESD procedure was performed on consecutive patients by a single endoscopist, which could reduce the bias in relation to the complete resection and complication rates. Hence, a prospective randomized study is warranted. The second limitation relates to the durations of the follow-up periods, which varied for each patient. Since the number of patients involved in this study was small and the duration of the follow-up period was relatively short, the long-term outcomes of ESD, namely, the recurrence and survival rates, could not be determined. Although there are no differences in short-term outcomes in gastric ESD on outpatients or inpatients, further study is needed with more patients and a longer follow-up. Finally, while we assumed that the costs for the inpatient group would be higher, we could not produce an unequivocal comparison of the costs associated with inpatient and outpatient ESD. The cost was affected by the medical insurance, whether endoscopic ultrasonography was performed or not, ward grade. We were unable to control all of these factors, because this was a retrospective study. In our hospital, ESD in an outpatient setting helped to achieve cost savings of approximately 10%; however, a future study should definitively quantify the difference in costs.
In conclusion, ESD was found to be a safe and effective method for the treatment of outpatients with small EGC or dysplasia within the antrum. No significant differences were found between the inpatient and outpatient groups with respect to the outcomes and complications.
References1. Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol 2006;41:929ŌĆō942. 17096062.
2. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228ŌĆō1235. 19249769.
3. Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol 2005;3(7 Suppl 1):S71ŌĆōS73. 16013003.
4. Messmann H, Probst A. Management of endoscopic submucosal dissection complications. Endoscopy 2009;41:712ŌĆō714. 19670140.
5. Sugimoto T, Okamoto M, Mitsuno Y, et al. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol 2012;46:124ŌĆō129. 21959325.
6. Japanese Gastric Cancer AssociationJapanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101ŌĆō112. 21573743.
7. Jeon SW, Jung MK, Cho CM, et al. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg Endosc 2009;23:1974ŌĆō1979. 18553202.
8. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225ŌĆō229. 11156645.
9. Conio M, Ponchon T, Blanchi S, Filiberti R. Endoscopic mucosal resection. Am J Gastroenterol 2006;101:653ŌĆō663. 16464227.
10. Jung MK, Jeon SW, Cho CM, et al. Hyperglycaemia, hypercholesterolaemia and the risk for developing gastric dysplasia. Dig Liver Dis 2008;40:361ŌĆō365. 18291734.
11. Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877ŌĆō883. 17140890.
12. Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract 2012;2012:875323. 22536221.
13. Higashiyama M, Oka S, Tanaka S, et al. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc 2011;23:290ŌĆō295. 21951088.
14. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331ŌĆō336. 19001058.
15. Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006;63:596ŌĆō601. 16564858.
16. Abe Y, Inamori M, Iida H, et al. Clinical characteristics of patients with gastric perforation following endoscopic submucosal resection for gastric cancer. Hepatogastroenterology 2009;56:921ŌĆō924. 19621730.
17. Kiriyama S, Oda I, Nishimoto F, Mashimo Y, Ikehara H, Gotoda T. Pilot study to assess the safety of local lidocaine injections during endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 2009;12:142ŌĆō147. 19890693.
18. Choi HS, Kim KO, Chun HJ, et al. The efficacy of transdermal fentanyl for pain relief after endoscopic submucosal dissection: a prospective, randomised controlled trial. Dig Liver Dis 2012;44:925ŌĆō929. 22824834.
Table┬Ā1Baseline Characteristics of Patients Having Tumors of the Gastric Antrum, Less than 2 cm Treated by Endoscopic Submucosal Dissection in the Inpatient and Outpatient Groups 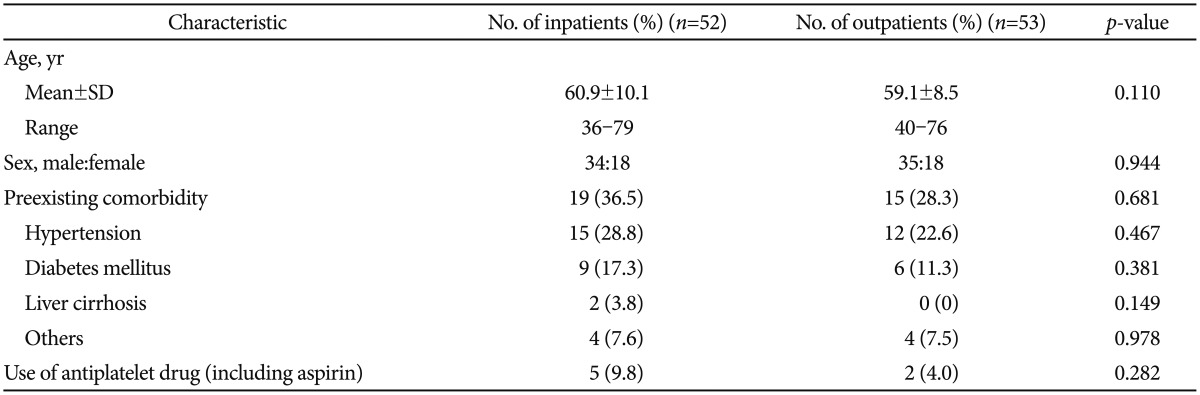
Table┬Ā2Clinicopathologic Features of Tumors of the Gastric Antrum Treated by Endoscopic Submucosal Dissection in Inpatients and Outpatients 
|
|
|||||||||||||||||||||||||||||||||||||||||||||||
