AbstractBackground/AimsEndoscopic mucosal resection (EMR) of large colorectal lesions is associated with increased procedural time. The objective of this study was to evaluate the effect of double-channel gastroscope (DCG) use on the procedural time of EMRs in the rectosigmoid area.
MethodsAll EMRs for sessile or flat rectosigmoid lesions Ōēź2 cm performed between July 2011 and September 2012 were retrospectively analyzed.
ResultsThere were 55 lesions Ōēź2 cm in the rectosigmoid area in 55 patients, of which 26 were removed by EMR using a DCG (DC group) and 29 by using an ordinary colonoscope or gastroscope (OS group). The mean size of the removed polyps, morphology, adverse effects, and other parameters were similar between the two groups. The mean procedural time was significantly lower in the DC group than in the OS group (24.4┬▒18.3 minutes vs. 36.3┬▒24.4 minutes, p=0.015). Moreover, in a subgroup of patients with polyps >40 mm, the statistical difference in the mean procedural time between the DC and OS groups was even more pronounced (33┬▒21 minutes vs. 58.7┬▒20.6 minutes, p=0.004).
INTRODUCTIONColonoscopy and endoscopic colonic polypectomy are considered the primary factors reducing colorectal cancer morbidity and mortality.1,2 Most of the polyps detected during colonoscopies are relatively small (<1 cm) and can be easily removed by conventional polypectomy techniques.3 Nevertheless, resection of larger (>2 cm) and especially flat (Ōēż2.5 mm elevated above the normal mucosa) or sessile polyps (Ōēź2.5 mm elevated above the normal mucosa)4 can be challenging even for experienced endoscopists.
Endoscopic mucosal resection (EMR), which was first described in 1955 for rigid sigmoidoscopy5 and then implemented in flexible colonoscopy by Deyhle et al.6 in 1973, is one of the most popular endoscopic techniques for the resection of large nonpedunculated colorectal polypoid lesions. En bloc removal of the polyp (in one piece) is considered ideal, as it renders an undivided histological specimen to the pathologist for a more accurate assessment; however, this is usually not feasible, especially in cases where the polyp is >2 cm in size and is therefore removed in a piecemeal fashion (more than one piece) by using EMR.7,8
The "inject-and-cut" technique in injection-assisted EMR, using snare and injection needle devices successively, has been proven to be safe and effective.9 Nonetheless, "inject-and-cut" EMR can become a technically demanding and particularly time-consuming procedure in cases with large or giant (>4 cm) polyps. The primary aim of our retrospective study was to evaluate whether the use of a double-channel gastroscope (DCG) for polyp EMR in the rectosigmoid area is related to a significant reduction in the procedural time compared to the use of an ordinary colonoscope or gastroscope (OS). The secondary aims were to record and compare other EMR parameters such as effectiveness and adverse effects of the procedure dependent on DCG or OS use. To the best of our knowledge, this is the first comparative study to evaluate the procedural time in EMR with the use of an OS or a DCG.
MATERIALS AND METHODSPatientsOver the last 2 years, a DCG has frequently been used for EMR of large left-sided nonpedunculated polyps in our department. Between July 2011 and September 2012, data on all EMRs of large sessile or flat rectosigmoid lesions >2 cm in size were retrospectively retrieved from an electronic database of patients who underwent colonoscopies at our department and were included in this study. All of our patients had undergone previous endoscopy using an OS, in which cecal intubation was achieved. This had taken place either in our unit or in other endoscopic units of our province, where the large left-sided polyps were detected and referred the patient to us for polyp resection, afterwards.
Written informed consent was obtained from all patients before all colonoscopies, and in cases of large polypectomies, a thorough explanation of the procedure was provided to the patients. Moreover, the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the ethics committee of our hospital. Colonic polyps were described according to the Paris classification of superficial neoplastic lesions.10
All patients receiving antiplatelet or anticoagulant drugs were asked to discontinue their medication at least 7 days before EMR. Bowel preparation was performed with a full dose of 4 L of polyethylene glycol (PEG) solution given on the day before the examination or with a split-dose of 2 L of PEG solution given on the day before and 2 L given on the day of the examination. According to our protocol, moderate sedation was administered with intravenous midazolam and pethidine right before endoscope insertion.11
Endoscopic mucosal resectionColonoscopy was performed by one experienced endoscopist (experience of over 15,000 colonoscopies). The examination was conducted using either an OS (CFH180AL, GIF Q165; Olympus Optical, Tokyo, Japan) or an operative DCG (GIF-2T160), and this was mainly dependent on instrument availability. Submucosal injection of a solution to lift the polyp was conducted using injection needles (Interjec, injection needle catheter; Boston Scientific, Natick, MA, USA), and the injected fluid was normal saline with adrenaline (1:10.000 dilution) and methylene blue for polyp staining. A standard snare (Master Snare/10 to 25 mm; Olympus Medical Systems, Tokyo, Japan) was used.
According to the "inject-and-cut" piecemeal EMR technique, the needle was inserted into the submucosal layer where the solution was diffused, creating a safety cushion and separating the muscularis propria from the submucosa. When the endoscopist was satisfied with the tissue elevation, the snare was opened and attached to the surface of the polyp, after which the endoscopist instructed the assistant to close the snare and encircle and resect a part of the mucosal lesion with (hot snare) or without (cold snare) electric current application. Whenever mucosal elevation subsided, the submucosal injection was repeated. This process was repeated several times until the mucosa was cleared endoscopically from the adenomatous tissue. A stopwatch was started at the time of the first submucosal injection and stopped after the last part of the polyp was resected; this time was recorded as the procedural time. Lesion's size was recorded based on pathology's report size in en bloc resections or endoscopist optical assessment in piecemeal EMRs. If the base of the lesion could not be lifted with solution injection after the largest part of the polyp was resected (nonlifting sign), submucosal cancer invasion was suspected and biopsy was performed.
Monopolar coagulation current using an ERBE ICC 200 generator (ERBE, T├╝bingen, Germany) with a setting of 120 W in "endocut" function was used. The diathermy for hot snare polypectomy was set at 120 W in endocut mode. At the end of the polypectomy or if the endoscopist was unsure about the complete removal of the polyp, argon plasma coagulation (APC) with ERBE APC 300 at a power of 40 to 70 W and gas flow of 2 L/min was performed at the rim of the polypectomy site for the complete removal of all adenomatous tissue inside the resected area of the polyp.12,13 All resected specimens were sent for histopathological evaluations to experts.
DefinitionsBleeding was defined as intraprocedural (at the time of polypectomy), early (within 24 hours), or delayed (24 hours after polypectomy). Postpolypectomy syndrome (PPS) was considered when localized abdominal pain, leukocytosis, and elevation of body temperature occurred within 24 hours after the procedure, without the presence of extraluminal air on radiological evaluation. Perforation was defined as the evidence of air or luminar contents outside the gastrointestinal tract on plain radiography or abdominal computed tomography (CT).14 Surveillance was recommended in all patients with noninvasive lesions. The first follow-up colonoscopy was performed within 3 to 4 months, and the second was performed at 12 months from polypectomy. In all follow-up endoscopies, biopsy specimens were obtained from polypectomy sites or scars. A residual polyp was defined as residual polyp tissue identified endoscopically and/or histopathologically at the polypectomy site during the first follow-up colonoscopy.15 Recurrence was defined as the presence of adenomatous tissue during the second follow-up colonoscopy and after endoscopic confirmation of polyp removal at the previous endoscopy.15 Patients with invasive cancer were referred to surgery.
Statistical analysisThe parameters evaluated were age, gender, polyp size, morphology, procedural time, adverse effects, histology, residual tissue, and recurrence. All calculations were conducted using the SPSS version 16.0 (SPSS Inc., Chicago IL, USA). Continuous data were compared using the unpaired Student t-test or Mann-Whitney U-test, as appropriate. Categorical variables were examined using the corrected chi-square or two-sided Fisher exact test. A value of p<0.05 was considered statistically significant. Variables that were significantly associated with the procedural time in the univariate analysis were entered into a multivariate stepwise linear regression model to identify the independent contribution of each factor. The threshold values for entry into and removal from the model were 5% and 10%, respectively.
RESULTSFifty-five patients who underwent EMR for nonpedunculated polyps in our endoscopy unit were included in this study. In 29 patients, an OS was used, and in the remaining 26 patients, a DCG was selected according to instrument availability. There was no significant difference in age, sex, Paris classification, polyp size, adverse effects, and the presence of residual or recurrent tissue in follow-up colonoscopies between the two groups (Table 1). The mean procedural time was significantly lower in the DCG group than in the OS group (24.4┬▒18.3 minutes vs. 36.3┬▒24.4 minutes, p=0.015) (Table 1). Moreover, subgroup analysis was performed in patients with polyp size Ōēź4 cm (n=26), and the difference in the mean procedural time between the DCG (n=14) and OS (n=12) groups was even more pronounced (33┬▒21 minutes vs. 58.7┬▒20.6 minutes, p=0.004) (Fig. 1).
Finally, multivariate stepwise linear regression analysis revealed that polyp size (╬▓=0.92, p<0.001) and use of a DCG (╬▓=15.5, p<0.001) were significantly associated with the proprocedural time (Table 2).
In our study, eight patients underwent en bloc removal of the polyp and the remaining 47 patients underwent piecemeal EMR. Most polyps were treated in a single session unless the endoscopist encountered events such as bleeding (one case) during EMR or abandoned the procedure (eight cases) owing to other reasons (depressed endoscopic image of the polyp or the large size of the polyp making complete resection difficult in a single session of colonoscopy). In those cases, if biopsy specimens from the resected polyp were negative for invasive cancer (n=5), repeat colonoscopy was rescheduled within less than a month. Of five patients showing a nonlifting sign, three tested positive for invasive cancer and were referred to surgery, one underwent transanal endoscopic microsurgery, and one underwent re-colonoscopy shortly and the residual polyp was removed.
There were five cases of bleeding (9.1%): four early (7.2%) and one procedural (3.8%). In one case of early bleeding, endoclips (Resolution Clip; Boston Scientific) and APC were used, and all the other cases resolved spontaneously without any medical intervention. There were four cases of PPS (7%), including two cases involving polyps in the rectum and two involving polyps in the sigmoid colon, which required an average hospitalization of 2.5 days and conservative treatment with broad-range antibiotics and nil by mouth. No case of perforation was observed. Nevertheless, two patients experienced localized pain in the lower abdomen after polypectomy, and CT conducted immediately revealed localized free air around the rectum. Both patients were treated conservatively.
The resected adenomas were divided according to their histological type and dysplasia grade.16 Histopathology of the resected polyps is presented in Table 3. At least two follow-up colonoscopies were performed in all cases. Fifteen patients (27.3%) and five (9.1%) experienced residual polyps and recurrent tissue, respectively, and they were treated using high-power APC,13 biopsy forceps, and polypectomy snares. All residual or recurrent polyps in patients were finally removed, and no patient was referred to surgery. Clinical management of all patients is presented in Fig. 2.
DISCUSSIONThe main outcome of this retrospective study is that the procedural time significantly reduced with DCG use, compared with OS use, in the resection of left-sided nonpedunculated lesions. This benefit seems to substantially increase with an increase in polyp size, especially in cases with very large polyps >4 cm in size, in which our results showed a considerable decrease in the procedural time with DCG use. DCG gives the option of placing both injection needle and snare in each of its working channels, using them tandemly according to endoscopist's willingness for injection or cut. This technical advantage makes this method convenient to use and reduces the polypectomy time, thus facilitating the endoscopist.
Moreover, compared with OS use in EMRs, DCG use provides similar safety and efficacy with regard to parameters such as polypectomy completeness, adverse effects, and recurrence rates. The most frequent adverse effect of EMR is bleeding, with a frequency of 1% to 15% described in the literature,8,17,18,19,20,21,22 which is consistent with the frequency of bleeding in the DCG group (7.7%) and the overall frequency of bleeding after EMR in the present study (9.1%).
The frequency of PPS in the DCG group was 3.8% and the overall frequency of PPS was 7.2%, and both values are consistent with the frequency of PPS reported in other previous studies (0% to 7.6%).8,17,18,19,20,21 Although the overall perforation rate after EMR is reported to be 0.7% to 4%, no case of perforation was observed in our study.8,17,18,19,20,21 Two patients showed localized free air around the rectum on CT, but this was due to APC implementation after polypectomy and was not considered as perforation but as a procedural incident.14 Both patients had undergone rectal polypectomies using an OS. Thus, although experts speculate that double-channel EMR may lead to an increased perforation rate, the data in our study do not support this view.23 The absence of perforation in our study may be a result of the small patient sample size as well as the location of the polyps in the left colon where the increased thickness of the bowel wall makes it difficult for perforation to occur as compared to other colonic sites.
Another significant drawback of large EMRs is the increase in the local recurrence rates, which reach up to 46% in some studies.8,17,18,19,20,21 Fifteen patients (27.3%; nine in the DCG group and six in the OS group) experienced residual polyps in the first follow-up colonoscopy. Recurrence was detected in five patients (9.1%; two in the DCG group and three in the OS group) in the second follow-up colonoscopy. The recurrence rates in the present study are comparable with those in previously published studies. Indeed, the difference between residual and recurrent polyps has not yet been clarified in the literature, which makes data interpretation slightly difficult. Nevertheless, after the third follow-up colonoscopy, all residual tissues were removed, and this was confirmed with both endoscopic and histological examinations.
There are certain limitations in our study. First, this was a retrospective study. Thus, the unblinded fashion of the study as far as the endoscopist is concerned, might influence the outcomes measures; however, this limitation is unavoidable. Second, our patient sample size was relatively smaller than that of other large studies, and this is mainly attributable to the restricted location of the polyps in the rectosigmoid colon. Third, this was a single-center study, which was another restricting factor for data analysis.
In conclusion, use of a DCG for resecting rectal or sigmoidal nonpedunculated polyps can be a very convenient technique for endoscopists as it could reduce the procedural time in large polypectomies. In endoscopic departments with heavy workload this can be a clinical meaningful issue by means of improving work rates without setting in jeopardy the quality of advanced endoscopic procedures performed, like large left-sided EMRs. Prospective randomized controlled studies are needed to verify our results.
References1. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977ŌĆō1981. 8247072.
2. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687ŌĆō696. 22356322.
3. Rex DK. Have we defined best colonoscopic polypectomy practice in the United States? Clin Gastroenterol Hepatol 2007;5:674ŌĆō677. 17544994.
4. Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570ŌĆō578. 15933932.
5. Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg 1955;70:120ŌĆō122. 13217613.
6. Deyhle P, Jenny S, Fumagalli I. Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention. Dtsch Med Wochenschr 1973;98:219ŌĆō220. 4684531.
7. Seewald S, Soehendra N. Perforation: part and parcel of endoscopic resection? Gastrointest Endosc 2006;63:602ŌĆō605. 16564859.
8. Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54:62ŌĆō66. 11427843.
9. Ferrara F, Luigiano C, Ghersi S, et al. Efficacy, safety and outcomes of 'inject and cut' endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion 2010;82:213ŌĆō220. 20588036.
10. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3ŌĆōS43. 14652541.
11. Rex DK. Review article: moderate sedation for endoscopy: sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther 2006;24:163ŌĆō171. 16842446.
12. Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc 2002;55:371ŌĆō375. 11868011.
13. Tsiamoulos ZP, Bourikas LA, Saunders BP. Endoscopic mucosal ablation: a new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc 2012;75:400ŌĆō404. 22154411.
14. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446ŌĆō454. 20189503.
15. Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc 2012;76:255ŌĆō263. 22657404.
16. Konishi F, Morson BC. Pathology of colorectal adenomas: a colonoscopic survey. J Clin Pathol 1982;35:830ŌĆō841. 7107955.
17. Arebi N, Swain D, Suzuki N, Fraser C, Price A, Saunders BP. Endoscopic mucosal resection of 161 cases of large sessile or flat colorectal polyps. Scand J Gastroenterol 2007;42:859ŌĆō866. 17558911.
18. Bergmann U, Beger HG. Endoscopic mucosal resection for advanced non-polypoid colorectal adenoma and early stage carcinoma. Surg Endosc 2003;17:475ŌĆō479. 12415335.
19. Bories E, Pesenti C, Monges G, et al. Endoscopic mucosal resection for advanced sessile adenoma and early-stage colorectal carcinoma. Endoscopy 2006;38:231ŌĆō235. 16528648.
20. Mahadeva S, Rembacken BJ. Standard "inject and cut" endoscopic mucosal resection technique is practical and effective in the management of superficial colorectal neoplasms. Surg Endosc 2009;23:417ŌĆō422. 18806938.
21. Su MY, Hsu CM, Ho YP, et al. Endoscopic mucosal resection for colonic non-polypoid neoplasms. Am J Gastroenterol 2005;100:2174ŌĆō2179. 16181365.
Fig.┬Ā1Polyp size and mean procedural time. DCG, double channel gastroscope; OS, ordinary gastroscope or colonoscope.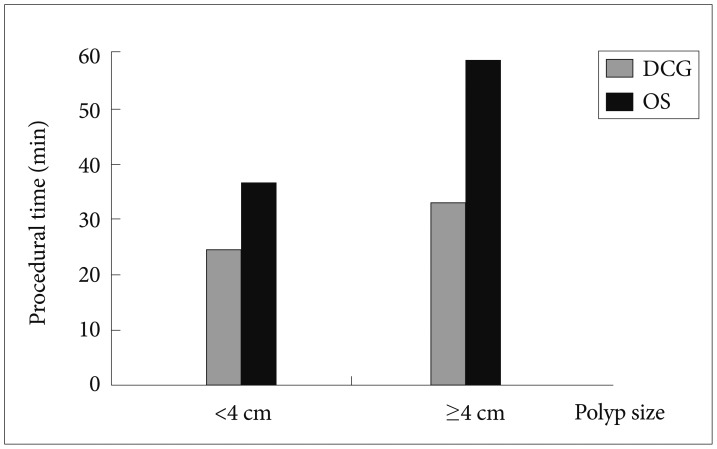
Fig.┬Ā2Clinical management of the patients. EMR, endoscopic mucosal resection; DCG, double channel gastroscope; OS, ordinary gastroscope or colonoscope; TEMS, transanal endoscopic microsurgery.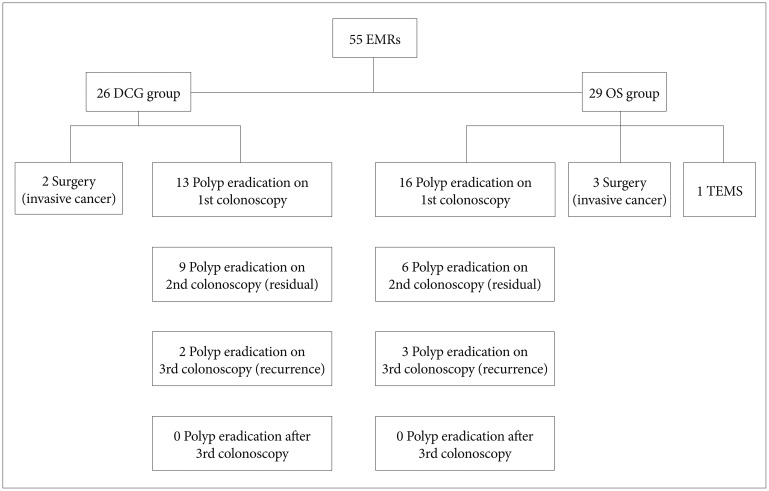
Table┬Ā1Comparison between Characteristics of Double Channel Gastroscope and Ordinary Gastroscope or Colonoscope Group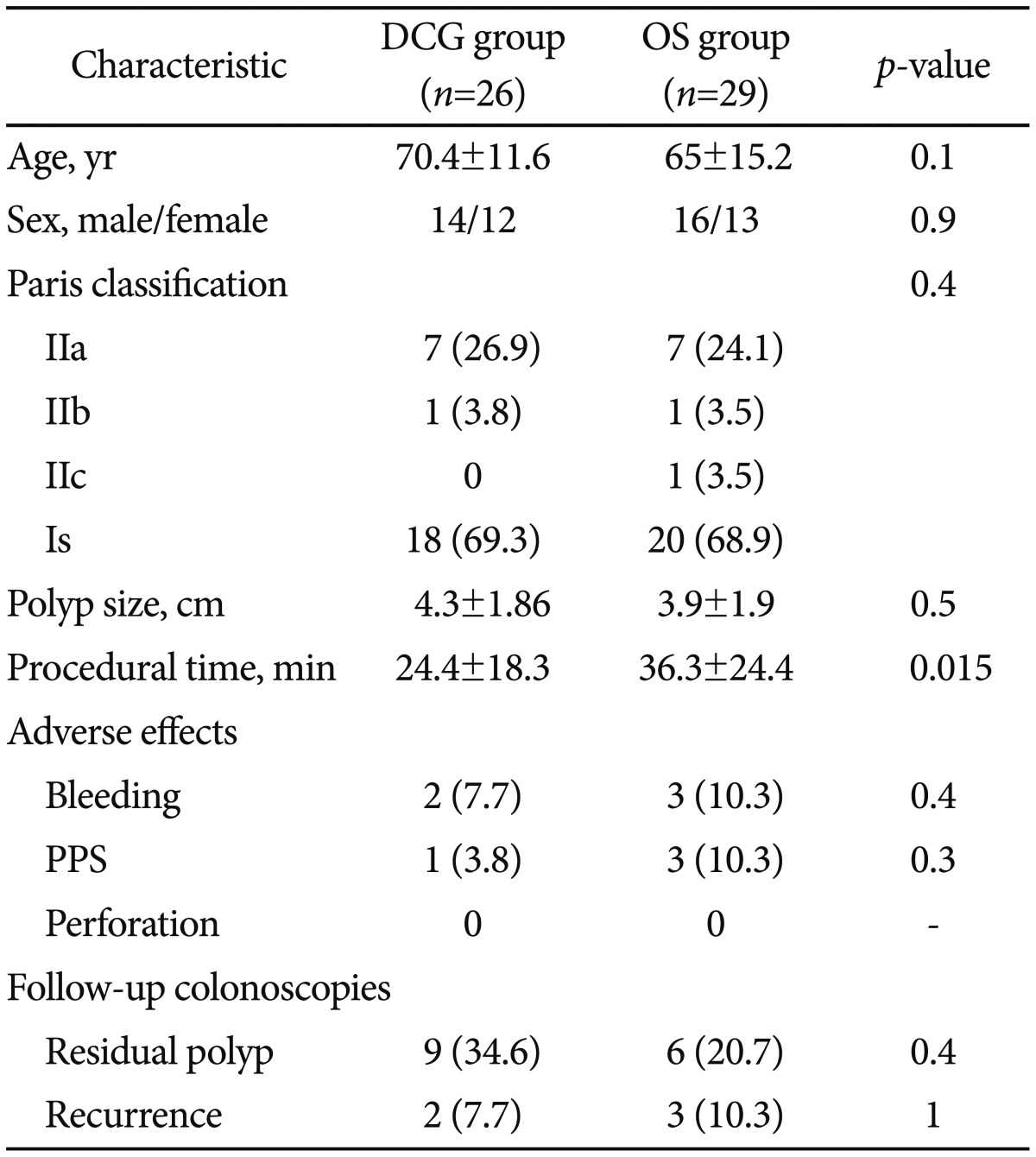
|
|
|||||||||||||||||||||||||||||||||||||||||||||||
