INTRODUCTION
Colon cancer is the third most common malignancy in Korea. Colonoscopy is considered the gold standard for detecting cancer and precancerous polyps in the colorectum, and it is the current standard procedure used to identify and remove adenomatous colon polyps [1-4]. Although it was initially estimated that colonoscopy with polypectomy can decrease the incidence of colon cancer by as much as 76% to 90% [1,5], several studies reported that a significant number of polyps are missed during routine colonoscopy [1,5,6]; furthermore, missed adenomas might reduce the preventive efficacy of colonoscopy against colon cancer [4,7,8]. Certain risk factors associated with missed polyps have been reported. Missed polyps were mainly associated with several factors, such as patient-related factors, polyp location, and the adequacy of bowel preparation. A recent prospective multicenter study [9] reported miss rates of 28% for polyps and 20% for adenomas of all sizes. The authors found that flat polyps and polyps in the right colon were associated with an increased polyp miss rate [4,9]. In addition, colonoscopy has inherent limitations, which may be both technical and operator dependent [10]. The operator-dependent nature of this procedure was well noted in several clinical studies that demonstrated different detection rates of colorectal cancers and adenomas between colonoscopists with different experience levels [11-13]. Therefore, the polyp detection rate (PDR) will be affected by the level of expertise of the colonoscopist [14,15], and flat lesions often remain undetected during regular standard white-light colonoscopy [16]. Previous studies that assessed whether the presence of an additional observerŌĆömore specifically, a gastrointestinal (GI) fellowŌĆöduring colonoscopy can enhance the detection of all polyps and adenomas have yielded conflicting results, and factors such as polyp size, location, and shape have not been rigorously evaluated [14,16-18]. Hence, in the present study, we aimed to evaluate the changes in the PDR in relation to the size, location, and shape of polyps with increasing experience in colonoscopy. Three fellows who were trained in colonoscopy participated in this study, and outpatients who wanted to undergo colonoscopy for screening purposes were enrolled.
MATERIALS AND METHODS
Data collection
The computerized clinical and endoscopic chart database of Inje University Haeundae Paik Hospital in Busan, Korea, served as the data source for this retrospective study. The computerized charts of patients who underwent colonoscopy between March 2010 and February 2011 in the general outpatient practice setting were reviewed. Patient information about age, sex, race, and family history of colon cancer was collected. The colonoscopic information collected included the quality of bowel preparation, indications for colonoscopy (screening, surveillance, or diagnostic), endoscopist, and withdrawal time. The characteristics of the polyps detected during colonoscopy (size, location, and shape) were also recorded. This study was approved by the Institutional Review Board of Inje University Haeundae Paik Hospital (2012-079).
Study population
The patients were scheduled to undergo a colonoscopy during a previous visit to the gastroenterology clinic from March 2010 to February 2011. The inclusion criteria were asymptomatic patients older than 50 years in whom screening colonoscopy was performed during the study period, with a withdrawal time of >6 minutes. The exclusion criteria were (1) emergency colonoscopy, (2) colon obstruction, (3) a history of colon operations, (4) a history of therapeutic procedures (including polypectomy), (5) surveillance of inflammatory bowel disease, and (6) a familial history of polyposis syndrome (familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, and juvenile polyposis). Three first-year GI fellows participated in this study. The starting level of training involved the observation of colonoscopies performed by seniors (at least 100 cases), and the training also included an understanding of the indications, performance of procedures, patient monitoring, use of sedatives and analgesics, interpretation of the findings, and avoidance of complications.
Colonoscopy information
All colonoscopies in our practice were performed in the white-light imaging mode as the primary method of detection. Endoscopists used adult video colonoscopes (CF-H260AL colonoscope; Olympus, Tokyo, Japan) with variable stiffness. Bowel preparation was uniform and consisted of 4 L polyethylene glycol; patients were instructed to ingest 2 L polyethylene glycol solution at 6:00 PM on the day before the procedure, to begin the ingestion of the remaining 2 L at 5 hours before the procedure, and to finish the ingestion of the dose at 3 hours before the colonoscopy on the day of the procedure. The patients received conscious sedation with intravenous midazolam plus meperidine. At this level of sedation, the patient is able to make a purposeful response to verbal or tactile stimulation, and both ventilatory and cardiovascular function are maintained. The starting dose of midazolam was 3 to 4 mg given slowly intravenously, and titrated to the desired effect by 1 mg every 2 to 3 minutes if needed. The usual total dose is 5 to 7 mg. Smaller doses (4 mg) may be used in the elderly. Larger doses (7 mg) may be necessary in some patients to achieve the desired effect. The usual dose of meperidine is 25 to 50 mg. Some patients may require larger doses (50 mg) to achieve the desired effect. The endoscopy nurse recorded the times for the following procedural events: insertion of the colonoscope into the rectum, identification of the base of the cecum, and withdrawal of the colonoscope across the anus, with times rounded to the nearest minute. We defined the colonoscopic insertion time as the time from insertion into the rectum to the identification of the base of the cecum, and the withdrawal time as the time from cecal identification to the withdrawal of the colonoscope across the anus. This withdrawal time excluded the time taken for maneuvers such as polypectomy that were performed during the withdrawal phase of the examination. The number, size, locations, and method of removal of the polyps were recorded by the fellow who performed the procedure. Bowel preparation quality was calculated by using the Ottawa bowel preparation scale [19], which assesses three components of the large intestine. The scale has a range from 0 (perfect) to 14 (a completely unprepared colon). Patients with scores >10 with inadequate bowel preparation were excluded from this study. We estimated the sizes of the lesions at the time of the colonoscopy through an in vivo comparison by using standard endoscopic instruments including open-biopsy forceps (7 mm, FB 24-U-1; Olympus). Colonoscopists were instructed to remove all the detected polyps.
Assessment of outcome
The primary outcome of this study included the change in the PDR in proportion to the accumulation of experience in colonoscopy. The secondary outcome included the change in the PDR in relation to the features that make polyps difficult to detect (e.g., <5 mm in size, flat shape, and location in proximal sites), which was based on an increase in the number of colonoscopies. Flat polyps were defined according to the definition of the Japanese Research Society Classification, which defines flat polyps as those with a height of less than one-half of the measured diameter [20,21]. Proximal sites were defined as the cecum, ascending colon, and transverse colon. In this study, the PDR was expressed in two ways. First, the percentage of patients who had polyps (even if only one polyp was found, the polyp was included in the PDR) was used as method A, and this method is commonly used in several existing papers [13,14,17]. With this method, one cannot perform an analysis that distinguishes between patients according to the number of polyps detected. In other words, although there was a difference in the detection rate according to the number of polyps, it is impossible to distinguish between two patients with different numbers of polyps by using method A; thus, representing a limitation of this method. Therefore, we attempted to compare the detection rates of polyps by using method B, in which the PDR was calculated as a percentage of the total number of polyps detected [13].
Statistical analysis
Patient characteristics were summarized by using descriptive statistical methods. Categorical characteristics were analyzed with the chi-square test for differences between groups, and continuous characteristics were analyzed with analysis of variance. The PDR was estimated, and the exact 95% confidence interval was also reported by using the Clopper-Pearson method. The numbers of polyps were summarized by using the mean┬▒SD and PROC generalized linear model, and a CONTRAST statement was employed for testing linear trends. A p<0.05 was considered statistically significant. The study was explorative in nature, and, therefore, no adjustment for multiple testing was made. All statistical analyses were conducted by using the statistical software SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
In total, 2,549 asymptomatic patients who underwent screening colonoscopy between March 2010 and February 2011 were enrolled in the study. The mean age of the patients was 54.9┬▒12.7 years. The patients included 1,279 men (50.2%) and 1,270 women (49.8%), and none had a history of surgery. The endoscopist-assessed quality of the bowel preparation was comparatively acceptable, and the mean Ottawa scale score was 4.7┬▒2.3 points. The patient characteristics and polyp detection data are summarized in Table 1. There were no significant differences in the number of cases and the age and sex of the patients treated by the three fellows. The total number of detected polyps was 889; polyps <5 mm were most common: 151 for A fellow, 136 for B fellow, and 187 for C fellow (p=0.0073). The tumor type was categorized as flat, pedunculated, semipedunculated, sessile, or lateral spreading. The location was divided into proximal and distal; the number of detected polyps in the distal colon was 110 for A fellow, 142 for B fellow, and 156 for C fellow (p=0.0154). There were no serious complications among the three groups. Statistical differences in the PDR were not observed among the three fellows.
Primary outcome
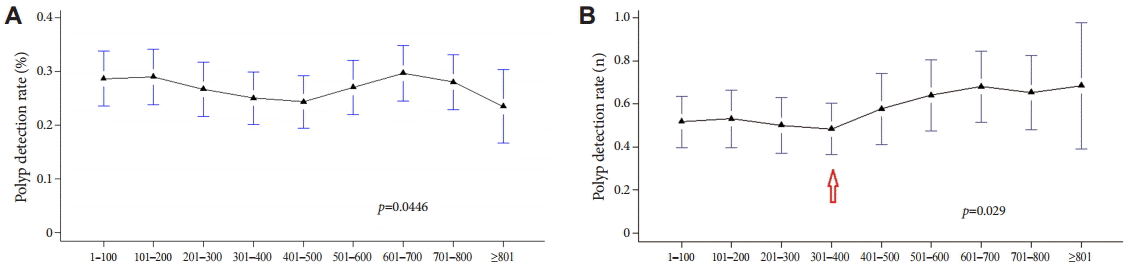
The PDR and adenoma detection rate (ADR) were compared separately 100 times (to the accumulation of experience in colonoscopy) and were also assessed among the three fellows (Table 2). Adenoma included the presence of tubular adenoma with low-, moderate-, or high-grade dysplasia; tubulovillous adenoma; villous adenoma; serrated adenoma; and adenocarcinoma in the histopathological examination. Table 2 presents a diagram obtained by using method A (the percentage of patients who had polyps). A comparison of the PDRs and ADRs from the three fellows revealed no statistically significant differences. Thus, the overall PDR and ADR, which were obtained by summing the data for all patients who had polyps in the three groups, are shown as a bar graph in Fig. 1. The PDR tended to decrease with increasing experience up to 500 procedures, followed by an increase up to 700 procedures, and a subsequent decline with increasing experience thereafter. The ADR declined with increasing experience up to 400 procedures, followed by an increase up to 700 procedures, and a subsequent decline with increasing experience thereafter (Fig. 1). Thus, when using method A to compare the PDR and ADR, no correlation between the detection rates and increasing experience in colonoscopy was observed, probably because method A did not differentiate between the number of polyps detected in patients. Thus, method A has a limitation in comparing the PDRs because the number of polyps detected is not considered. Consequently, the PDR was calculated by using method B, which accounts for the total number of polyps detected. Fig. 2 presents a comparison of the PDRs calculated with the two methods per 100 colonoscopies. Because the procedures were summed for the three fellows, each increment in the colonoscopies by 100 included a total of 300 procedures. As stated previously, the PDR did not display a proportional relation to experience in colonoscopy with method A (Fig. 2A). However, with method B, although the PDR was not uniformly increased with increasing procedural experience (Fig. 2B), a significant increase was observed with experience of >400 colonoscopies (p=0.029).
Secondary outcome
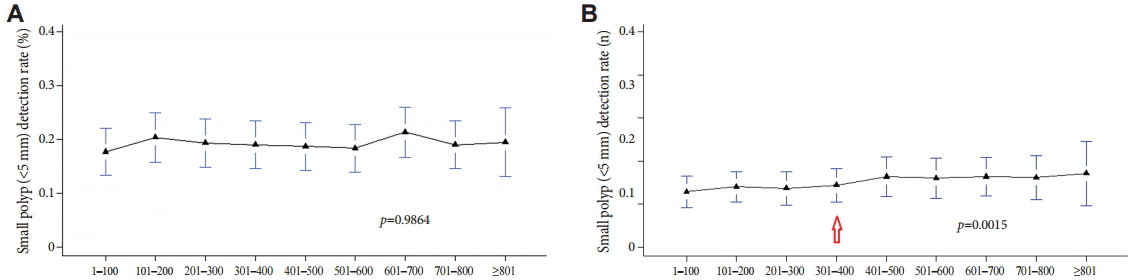
To determine whether there was an association between the detection rate of polyps with characteristics that make them difficult to detect and the level of experience in colonoscopy, the PDR was calculated by evaluating the detected polyps according to size (<5 mm or larger), shape (flat forms were more difficult to detect), and location (polyps in proximal sites were easier to miss). A correlation between the detection rate of polyps <5 mm and experience with colonoscopy was rarely observed with method A (Fig. 3A); with method B, as shown in Fig. 3B, the detection rate of polyps <5 mm increased slightly, but significantly, with an accumulation of experience of >300 colonoscopies (p=0.0015).
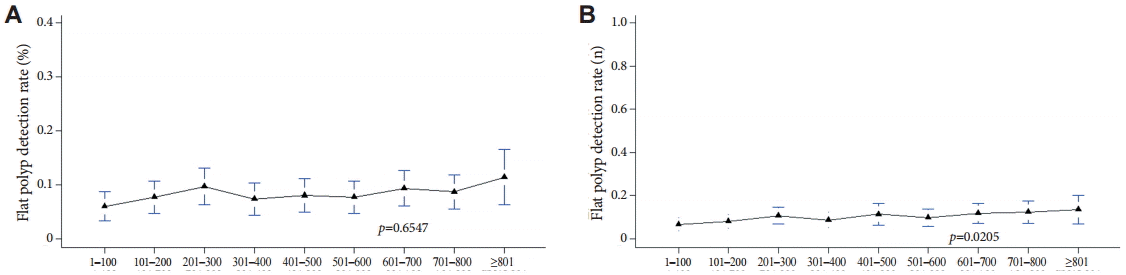
With increasing experience with colonoscopy, the detection rate of flat polyps did not increase with either method A or B (Fig. 4).
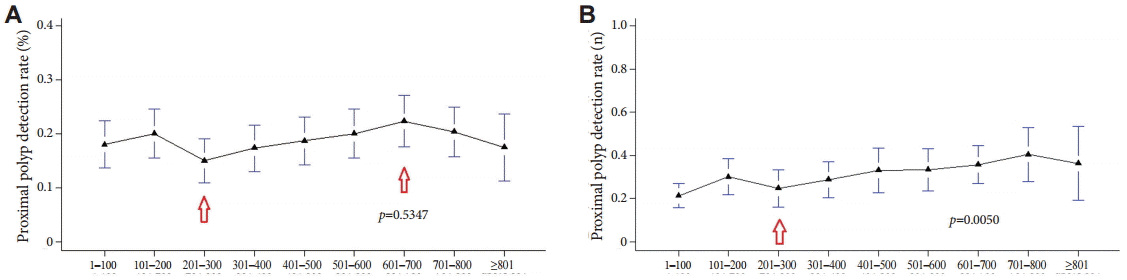
For the right colon, where the rate of missed polyps is known to be high, we compared the PDRs for the proximal colon by using the two methods. The PDR determined with method A began to increase steadily after 200 procedures, although it declined slightly after 700 procedures (Fig. 5A). It was not possible to clarify the proportional relation between the PDR and increased experience in colonoscopy (p=0.5347). On the other hand, with method B, the PDR for proximal sites was not constant over time but increased steadily after 300 colonoscopies (Fig. 5B); moreover, the observed difference was statistically significant (p=0.0050).
DISCUSSION
Colonoscopy is recognized as the most effective method of colon screening in patients at risk of certain conditions, considering that colonoscopy and polypectomy have effectively reduced the incidence of colorectal cancer [1,2]. There have been many studies on the learning curve of GI fellows, and these studies concluded that fellows should perform at least 100 to 200 procedures to be considered technically competent in diagnostic colonoscopy [22-24]. A fellowŌĆÖs colonoscopy success rate depends on the number of colonoscopies completed [15]. The success rate significantly improves and reaches the standard of competence (>90%) after 150 procedures [15]. In fact, the earliest level at which any of the fellows reached the >90% colonoscopy independence rate was 330 colonoscopies, and all fellows achieved a 90% colonoscopy independence rate by 500 colonoscopies [25]. This finding suggests that 500 colonoscopies may be a more appropriate minimal threshold to ensure competence, if competence is solely defined as a >90% colonoscopy independence [25]. However, considering these data, how does the PDR change after the threshold of the learning curve is reached? To our knowledge, a study on the correlation between the learning curve and PDR has not been performed. The PDR is the most widely used means of measuring the quality of colonoscopy, and, in the present study, we aimed to establish the effect of a GI fellowŌĆÖs expertise in performing screening colonoscopies. We observed that the PDR increased with increasing experience of the fellow, and with an experience of >300 colonoscopies, small, clinically nonsignificant polyps and polyps deemed difficult to detect could be detected at higher rates. The fact that the PDR increased with higher levels of training suggests that the colonoscopistŌĆÖs expertise is an important factor in the quality of screening colonoscopy. Thus, colonoscopy can be considered a technically demanding procedure that requires considerable training and experience for optimal performance. Furthermore, caution must be exercised when assessing the data for fellows with an experience of >800 procedures, as there were only 149 cases available instead of 300.
This study has several strengths. The retrospective design eliminates the possibility of observer bias (gastroenterologists might be more aggressive than usual in polyp detection when they are aware that their detection rates are being recorded). Misclassification of polyps was unlikely because every screening colonoscopy report was reviewed. Colonoscopy withdrawal times have been demonstrated to strongly affect the PDR [12,13], and the withdrawal phase for colonoscopy should average at least 6 to 10 minutes, according to an observational study [26]. It is possible that slower withdrawal times could explain the observed differences in detection rates. Therefore, only colonoscopies for which the withdrawal time exceeded 6 min were assessed in this study [13].
This study has some limitations. First, this was a single-center study of procedures performed by three fellows. Therefore, these results are not adequate to permit generalization, and interobserver variation may exist. To minimize these limitations, we conducted frequent study meetings and provided standardized instructions for assessment. Moreover, three first-year GI fellows with less experience were included in this study; to achieve clinical significance, three groups of endoscopists who were classified on the basis of their year of training (first, second, or third) should be compared. Second, the enrolled patients were outpatients without significant medical problems, and we excluded patients who underwent colorectal surgery. Considering that colonoscopy is performed mostly in outpatients in our country, we conducted the study in an outpatient setting. The results of our study require further confirmation through multicenter randomized trials including an unselected group of patients. Third, multiple factors have been implicated in polyp detection, including colonoscopy imaging techniques; the participation of an experienced nurse; patient sex, age, and body mass index; and the time when the procedure is performed. As examination techniques improve, the PDR is likely to increase [26-28]. In this study, we exclusively used wide-angle (170┬░), high-definition colonoscopes that may have contributed to our average PDR, although evidence addressing the advantages of these technologies is limited [29-33]. A recent study by Dellon et al. [34] indicated that the experience of the endoscopy nurse influences PDRs, as the detection rate is lower for colonoscopies involving less experienced nurses. The incidence of polyps has been found to correlate with increased age and male sex [35,36], and patient body mass index has an association with preparation quality [37,38]. A number of studies evaluated the influence of the time of day on colonoscopy performance, including polyp detection and cecal intubation rates, and most studies reported an inverse association between a later time and the PDR [39,40]. We could not control for various factors that affected the PDR in our analyses. Therefore, this study should not account for the observed differences in PDR without error.
In conclusion, this study demonstrated that the expertise of GI fellows influences the PDR during screening colonoscopies. Furthermore, the level of fellow training clearly affects the detection rates for small (<5 mm) and proximal polyps, with higher rates observed at an experience level of >300 colonoscopies. This study suggests that in addition to technological advances and improvements in the field of view and imaging, ensuring that the procedure is performed by a trained fellow might greatly improve the PDRs. However, future prospective studies are needed to determine the factors that affect the PDR.