Endoscopic Closure for Full-Thickness Gastrointestinal Defects: Available Applications and Emerging Innovations
Article information
Abstract
Full-thickness gastrointestinal defects such as perforation, anastomotic leak, and fistula are severe conditions caused by various types of pathologies. They are more likely to require intensive care and a long hospital stay and have high rates of morbidity and mortality. After intentional full-thickness opening of hollow organs for natural orifice transluminal endoscopic surgery, safe and secure closure is urgently required. The currently available advanced endoscopic closing techniques have a major role in the treatment of full-thickness gastrointestinal defects. Appropriate usage of these techniques requires taking into account their advantages and limitations during practical application. We reviewed the available endoscopic modalities, including endoscopic clips, stents, vacuum-assisted closure, gap filling, and suturing devices, discussed their advantages and limitations when treating full-thickness gastrointestinal defects, and explored emerging innovations, including a novel endoluminal surgical platform for versatile suturing and a cell-laden scaffold for effective gap filling. Although these emerging technologies still require further pre-clinical and clinical trials to assess their feasibility and efficacy, the available modalities may be replaced and refined by these new techniques in the near future.
INTRODUCTION
Full-thickness gastrointestinal defects such as perforation, leak, and fistula arise from various causes and frequently require admission to the intensive care unit and a long hospital stay [1-5]. Historically, full-thickness gastrointestinal defects have been treated with a combination of reoperation, drainage, antibiotic therapy, and total parenteral nutrition [6-8]. However, despite the availability of these options, these defects are still strongly associated with a high rate of morbidity and mortality. For example, mortality rates for anastomotic leak after esophagectomy have been reported to range from 30% to 60% [9-11].
In the field of minimally invasive surgery, natural orifice transluminal endoscopic surgery (NOTES) is an emerging endoscopic procedure performed in the abdominal cavity through full-thickness openings of hollow organs. Planned perforations for NOTES access require a safe and secure full-thickness closure to reduce the risk of dehiscence and intra-abdominal abscess. Therefore, management of full-thickness defects is necessary for NOTES procedures as well.
With the currently available applications, it is possible to manage gastrointestinal defects without surgery. However, there are some limitations and disadvantages associated with each of the procedures. Here, we review the available endoscopic modalities for the management of gastrointestinal defects, including endoscopic clips, stents, vacuum-assisted closure (VAC), gap filling, and suturing devices, and explore the emerging innovations.
ENDOSCOPIC CLIPS
Through-the-scope clips (TTSCs) are introduced through the biopsy channel. They were first designed for the management of gastrointestinal bleeding. Recently, TTSCs have been used for the closure of gastrointestinal perforations [12,13]. These clips are preferred because of their ease of use and rotatable, re-openable, and off-the-shelf features. TTSCs have been reported to be successful for iatrogenic perforations and fistulas in the gastrointestinal tract, with success rates ranging from 59% to 83% [14,15]. The limitations of TTSCs are their smaller size and smaller closing force.
Over-the-scope clips (OTSCs; Ovesco Endoscopy, Tübingen, Germany) enable closure of full-thickness defects measuring 2 cm or smaller in diameter [16]. A twin grasper and a tissue anchor are quite useful for pulling the tissues into the cap and reducing the size of the gap before deployment. Due to their larger size and larger closing force, OTSCs can close large defects and achieve full-thickness bites of the surrounding tissues. OTSCs have been reported to be successful for closing gastrointestinal defects, with long-term success rates ranging from 71% to 100% [17-21]. One retrospective study of patients with acute perforations, leaks, and fistulas treated with OTSCs reported long-term success rates of 90%, 73%, and 43%, respectively [22]. Chronic leak and fistula with inflammation are believed to be the main reasons for closure failures with OTSCs [23]. Care must be taken when introducing OTSCs because their bigger size can sometimes cause iatrogenic perforations [24].
The combination of TTSCs and a detachable snare (MAJ-340; Olympus Optical Co., Miami, FL, USA) has recently been proven as a promising option for closing larger gastrointestinal defects. This combination was first developed with a two-channel endoscope for larger mucosal defects after endoscopic resection [25]. A detachable snare is initially placed around the defect. Then, TTSCs are applied to the snare with the surrounding tissues to fix them around the defect. Subsequently, the detachable snare is tightened to close the gap [26]. However, this method is not always useful in some situations, and the success rate was only 61% in this study [25].
STENTS
It is common to use stents to cover full-thickness defects in the stomach after bariatric surgery and in the esophagus and colon. Stent placement is effective due to the diversion of enteric contents away from the defect. Various types of stents have been used, such as metallic (partially or fully covered), plastic, and biodegradable. Stent deployment often enables continuation of oral intake and can be useful for larger defects [ 7,27,28]. However, stents tend to migrate in 20% to 30% of cases lacking stenosis and thus require frequent radiographic observation [28,29]. Another concern regarding this procedure is the necessity of removal; appropriately timing endoscopic removal can sometimes be difficult. In a study of patients with esophageal leaks, the success rate was 85% and there were no significant differences between plastic stents and fully or partially covered metal stents [30]. The migration rates were higher for plastic stents and fully covered metal stents compared to partially covered metal stents (31%, 26%, and 12%, respectively). Partially covered stents allow tissue in-growth, and this phenomenon can prevent stent migration; however, it also can interfere with safe endoscopic removal. A meta-analysis of seven studies regarding leaks after bariatric surgery reported a success rate of 88% and a migration rate of 17% [31]. The use of biodegradable stents for patients with esophageal leaks was reported in another study [32]. The success rate was 80%, but the migration rate was 60% during follow-up. Additional clip placement or suturing to prevent migration may be useful. One study reported that additional clips to anchor the stents were useful; migration rates were 13% for patients with additional clips and 34% for patients without them [33]. Another study regarding esophageal leaks mentioned the possibility of extension of the anastomotic dehiscence or the erosion of the stent into the trachea and large vessels [34].
VACUUM-ASSISTED CLOSURE
VAC has been used for esophageal and colorectal leaks. VAC therapy is performed with a porous polyurethane sponge mounted at the tip of a gastric tube. This device is introduced using endoscopic forceps and is placed into the defect. Then, controlled, continuous, negative pressure is applied. The sponge needs to be changed every 2 to 5 days; it continuously removes wound secretions and interstitial edema. To achieve improved micro-circulation and granulation of the wound, the healing process is promoted [35]. One study regarding VAC therapy reported that the success rate was 93% for patients with esophageal leaks [36]. In another study of five patients with esophageal leaks, the success rate of VAC therapy was 100% and the median length of the treatments was 28 days with nine sponge changes [37]. Two of these five patients presented with stenosis and one experienced severe bleeding after endoscopic dilation due to an aorto-anastomotic fistula.
GAP FILLING
Fibrin glue and cyanoacrylate are sealants that have been used to fill gastrointestinal defects [38,39]. Fibrin glue, a biologic sealant, is composed of fibrinogen and thrombin. It is applied with a double lumen catheter and then combined to form an acellular clot in the defects. Fibrin glue injected submucosally has been reported to have caused a wheal and subsequent occlusion of a tracheoesophageal fistula [40]. Another study of 15 patients with a gastrointestinal fistula reported that fibrin glue was used to fill the fistula; on average, the success rate was 87% after 2.5 sessions [38]. Another report mentioned a success rate of only 50% for cases of severe inflammation [41]. Cyanoacrylate, a synthetic sealant, has the advantage of strong adhesive and antibacterial characteristics. Therefore, it is considered suitable for application in infectious or wet environments and has been reported to be successful for closing an esophagojejunal anastomotic leak [39].
Surgisis (Cook Surgical, Bloomington, IN, USA) is an acellular bioactive prosthetic biomatrix derived from small intestinal submucosa of sheep [42]. This device was developed for the treatment of anal fistulas and has been used to successfully treat gastrocutaneous fistulas after bariatric surgery [43]. In one study, Surgisis was used endoscopically to occlude gastrointestinal fistulas; it had an 80% long-term success rate [44]. Vicryl plug in combination with fibrin glue has been successful in 87% of patients with gastrointestinal defects after surgery for esophageal cancer [45]. The number of sessions required for Vicryl plug and fibrin glue applications ranges from one to four. However, the author mentioned that it is best to apply this method when the size of the defect has decreased to 1.5 cm and when the site appears clean on lavage.
SUTURING DEVICES
The Overstitch Endoscopic Suturing System (Apollo Endosurgery, Austin, TX, USA) is a suturing device mounted at the tip of a double-channel endoscope. This device enables placement of full-thickness sutures and multiple uses without endoscopic removal. It has been used to close acute perforations as well as ulcerations after endoscopic resection [46,47]. In one study using a treat-and-resect model, the Overstitch system was able to place sutures consistently at a subserosal depth in the colon without injury to the surrounding organs [48]. It has also been successfully used for the closure of leaks after bariatric surgery and esophagopleural and gastrocutaneous fistulas [49-52]. However, in another study of patients with gastrogastric fistulas that were closed using another suturing device (EndoCinch; CR BARD, Billerica, MA, USA), the long-term success rate was 35%, despite a 95% initial closure rate [53]. Furthermore, another animal study reported that the procedure time for endoluminal closure was 1 hour, on average, even though the team had experience using this technique [54]. These findings suggest lingering issues regarding technical difficulty.
EMERGING INNOVATIONS
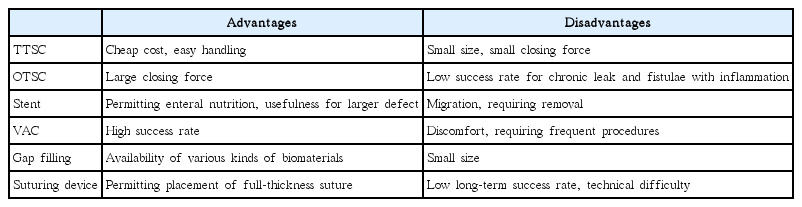
Although a number of options are available for repairing gastrointestinal defects, each still has several limitations and disadvantages (Table 1). A method for substantial and durable full-thickness closing of large defects is still required. We believe that the key items needed for successful closure are a versatile suturing device and effective gap-filling material.
Our group developed the master and slave transluminal endoscopic robot (MASTER) as a novel endoluminal surgical platform. It has two operating arms equipped with multiple degrees of freedom; thus, enabling dexterity with surgical maneuvers such as triangulation, retraction, grasping, and cutting [55]. Using this platform, we will be developing an intuitive suturing method characterized by adjustability and versatility (Fig. 1). This platform can also be combined with existing methods such as endoscopic clips. By means of grasping and traction, the MASTER system can provide a suitable situation for clip application (Fig. 2). With regard to gap-filling materials, cell-laden scaffolds that have been accepted in the skin or orthopedic area are promising for treating gastrointestinal defects [56,57]. We believe that gastrointestinal fibroblasts cultured on biodegradable and biocompatible materials (e.g., polycaprolactone) fabricated using three-dimensional printing techniques will prove suitable for implants to fill the defects in terms of growth efficacy and secretion of growth factors (Fig. 3).

Master and slave transluminal endoscopic robot suturing with adjustability and versatility in a dry setting.

Collaboration between master and slave transluminal endoscopic robot and endoscopic clips in an animal experiment. The grasper holds the defect and provides a suitable situation for clip application.
CONCLUSIONS
Gastrointestinal defects can be managed without surgery, but we still need further innovations and new technologies to achieve ideal clinical outcomes. Although the emerging technologies demand further pre-clinical or clinical trials to assess their feasibility and efficacy, the presently available applications may eventually be replaced and refined by these new techniques in the near future.
Notes
Conflicts of Interest: K.Y.H. is a co-founder of EndoMaster Pte Ltd.
Acknowledgements
The authors thank Wu Bin and Professor Jerry Fuh, Department of Mechanical Engineering, National University of Singapore, for providing the polycaprolactone scaffolds.