Percutaneous Transhepatic Cholangioscopy in Bilioenteric Anastomosis Stricture
Article information
Abstract
Bilioenteric anastomosis strictures are a serious complication of biliary surgery, and often result in recurrent cholangitis, choledocholithiasis, biliary cirrhosis, and hepatic failure. Bilioenteric reconstructive surgery is the standard treatment of choice for such complications. However, percutaneous transhepatic cholangioscopy (PTCS), also known as per-oral endoscopic-guided intervention, is a less invasive procedure that is becoming an increasingly popular alternative. This review describes the PTCS procedure (including the preparation process), as well as the diagnostic and therapeutic role of PTCS in bilioenteric anastomosis strictures.
INTRODUCTION
Bilioenteric anastomosis strictures may be caused by benign conditions such as postoperative fibrotic change and scarring, or malignant conditions such as tumor recurrence [1-3]. They are a serious complication of biliary surgery, and may result in recurrent cholangitis, choledocholithiasis, biliary cirrhosis, and hepatic failure. Bilioenteric reconstructive surgery is performed for patients with bilioenteric anastomosis stricture complications. However, this surgery is difficult to perform, and its success rate decreases with each successive surgery [2,4-7]. The percutaneous transhepatic cholagioscopy (PTCS) or peroral endoscopy-guided intervention is less invasive procedure that has become an increasingly popular alternative for managing bilioenteric anastomostic strictures. With the increase in the number of liver transplantations—especially living-donor transplantations—over the last few decades, the therapeutic role of PTCS has become even more important.
PROCEDURES
PTCS-guided interventions are laborious procedures, which require multi-step preparation before cholangioscopic intervention can be performed. First, percutaneous transhepatic biliary drainage (PTBD) is performed with an 8.5-Fr catheter inserted under fluoroscopic guidance into the right posterior branch or left peripheral branch of the intrahepatic duct, based on a planned anatomical approach to the stricture. Two or 3 days after PTBD, the percutaneous tract is dilated to allow for the passage of the cholangioscope. This is usually done by one-stage dilation (e.g., from 8.5 to 16 or 18 Fr) or stepwise dilation in two sessions. For sinus tract maturation, a 16- or 18-Fr PTC catheter is placed at least 7 days before cholangioscopy. Cholangioscopy is then performed using a 5-mm diameter cholangioscope [8,9].
Procedure-related complications tend to occur during PTBD insertion (12.9%), tract dilation (12.8%), and tract maturation (6.9%). Cholangitis and bacteremia are the most frequent complications. The incidence of complications for therapeutic cholangioscopies has been reported to be 6.7%, of which cholangitis and bile duct injuries are the commonest complications [9].
CHOLANGIOSCOPY
Diagnostic role
It is essential to be able to diagnose a bilioenteric anastomosis stricture accurately, because the choice of treatment depends on whether the cause is benign or malignant. Tissue acquisition is the mainstay of diagnosis. However, in bilioenteric anastomosis strictures, access is limited by the surgically altered anatomy and the limited visualization of the stricture site via a retrograde approach. As such, it is almost impossible to obtain a tissue sample with endoscopic retrograde cholangiopancreatography or single (or double) balloon enteroscopy. Instead, PTCS can play an important diagnostic role by allowing the direct visual inspection of a bilioenteric anastomotic lesion in an antegrade approach [10].
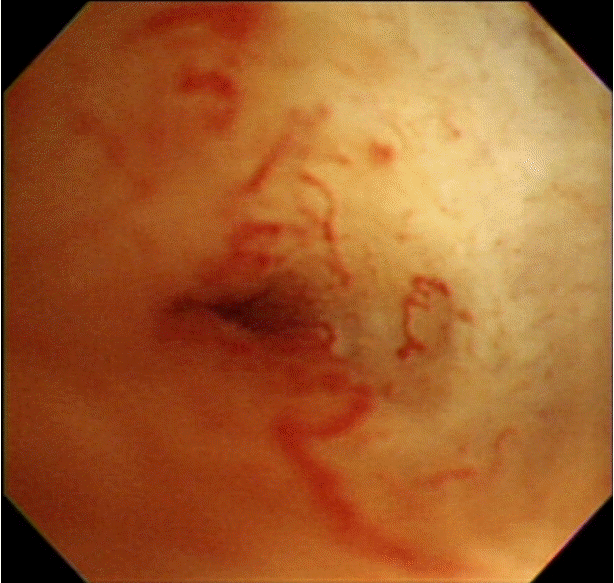
The typical cholangioscopic findings of a benign biliary stricture include the following: smooth mucosal surface, tapered luminal narrowing, short stricture segment (accompanying stones), and the absence of definite neovascularization (tumor vessel) [11]. A tumor vessel is an abnormally proliferating and tortuous vascular structure on the bile duct mucosa, and is one of the defining characteristics of biliary malignancy (Fig. 1) [12]. PTCS-guided target biopsies allow the direct visualization of tumor vessels. They were found to improve diagnostic accuracy significantly, especially in infiltrative cholangiocarcinoma. They had a sensitivity of 96%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 91% [12].
Therapeutic role
PTCS has several advantages over percutaneous cholangiography-guided interventions in treating bilioenteric anastomosis strictures. Due to the presence of bile stasis and remnant suture materials, postoperative bilioenteric anastomosis strictures are often complicated by bile duct stones. Large stones proximal to the stricture site can be easily fragmented with electrohydraulic shock-wave lithotripsy or laser lithotripsy, and then removed during stricture dilation. In addition, since remnant suture materials or surgical clips can act as niduses for stone formation, it is essential to remove any foreign bodies present at the bilioenteric anastomosis stricture site [13,14].
The main role of PTCS in bilioenteric anastomosis strictures is the recanalization and dilation of the strictures. Due to postoperative fibrosis and scarring, the strictures are often pinpoints (Fig. 2). PTCS guides the targeted recanalization with a guidewire or needle-knife [15,16]. The initial technical success rate was reported as 100%; however, the recurrence rate was 38% at a mean of 17 months. The overall success rate after repeated dilation was 82% after a mean follow-up period of 33 months, and the 5-year cumulative patency rate was 48%. There were no significant correlations between recurrence rate and the duration of catheter maintenance or number of sessions required [17].
One drawback of PTCS is that the working channel has a small diameter, and as such, a self-expandable metal stent cannot be placed through it. One study compared the temporary percutaneous placement of retrievable fully-covered metal stents with percutaneous balloon dilation in patients with benign biliary strictures. It found that the patency rates were significantly better in the metal stent group than in the balloon dilation group at 3 years (87% vs. 44%, p=0.022). The indwelling period of the PTBD catheter after the initial procedure was also shorter in the metal stent group (2.5 months vs. 4.5 months, p=0.001) [18].
PTCS is also useful in managing intractable bilioenteric strictures after balloon dilation. Although the evidence is limited to case reports, steroids (triamcinolone) can be injected directly into strictures refractory to treatment with balloon dilation and indwelling catheters [19]. For cases in which conventional methods for recanalization and/or dilation fail, PTCS can be used to guide the correct placement of magnets into the stricture site. Magnetic compression anastomosis was reported to be successful in 15 of 19 patients who had treatment-refractory benign biliary strictures or duct-to-duct anastomosis strictures after liver transplantation. Recanalization was achieved and maintained in all 15 patients at 1 year postprocedure [20,21].
CONCLUSIONS
Despite the laborious preparations required, PTCS has an important role to play in the management of bilioenteric anastomosis strictures.
Notes
Conflicts of Interest: The author has no financial conflicts of interest.