Role of Malabsorptive Endoscopic Procedures in Obesity Treatment
Article information
Abstract
The incidence of obesity is increasing, and more definitive treatment modalities are needed. Endoluminal procedures, including restrictive endoscopic procedures, endoscopic gastroplasty, and malabsorptive endoscopic procedures, can reduce weight in obese patients and control obesity-related comorbidities. Malabsorptive endoscopic interventions also offer the potential for an ambulatory procedure that may be safer and more cost-effective compared with laparoscopic surgery. Malabsorptive endoscopic intervention can induce weight reduction and improve obesity-related metabolic parameters, despite complications such as device migration, obstruction, and abdominal pain. Improvement in technique will follow the development of new devices.
INTRODUCTION
Gastric bypass surgeries are effective in weight loss and the control of combined comorbidities such as hypertension, diabetes, and obstructive sleep apnea. However, intra- and postoperative complications can occur [1]. Recently, new, less-invasive endoscopic techniques have been developed. At present, these interventions are useful for patients with mild obesity who do not have indications for surgery, and can also serve as a bridge to surgery to decrease obesity-related surgical risks. These procedures are primarily used to address comorbid illness such as diabetes, and have outcomes similar to those of traditional bariatric surgery, but with reduced procedure-related risks. They can also be used as revision procedures to manage failed bariatric surgery [2].
Among endoscopic procedures, a malabsorptive strategy offers control of obesity-related comorbidities as well as weight loss. Malabsorptive endoscopic procedures imitate the effects of gastric bypass surgery. Several procedures have been developed, including use of duodenal-jejunal bypass and gastroduodenal-jejunal bypass sleeves.
TYPES OF MALABSORPTIVE ENDOSCOPIC PROCEDURES
Duodenal-jejunal bypass sleeve
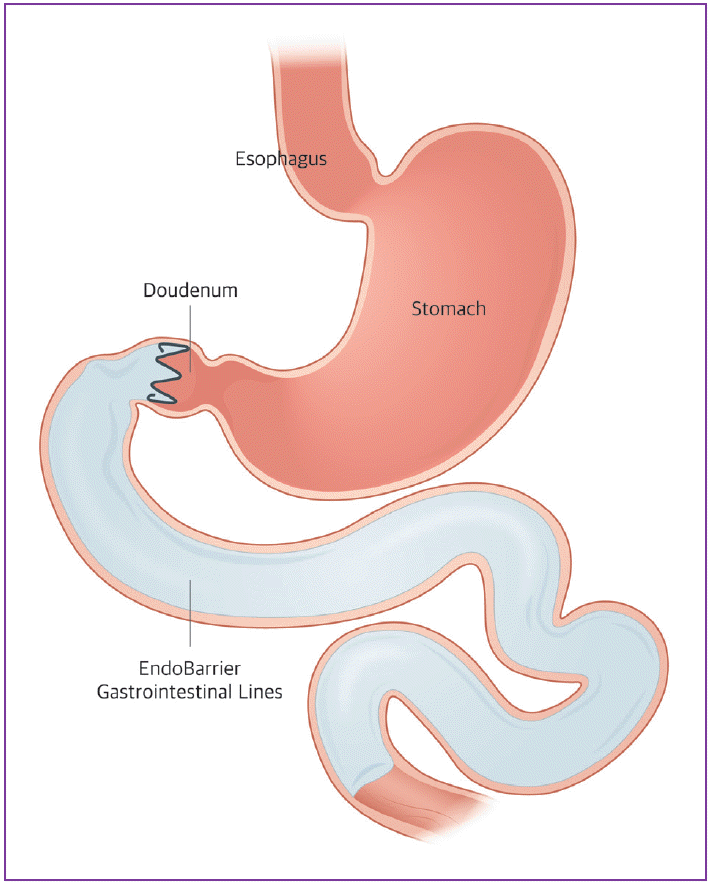
This method uses a flexible and removable 60-cm sleeve that is attached to the duodenum and bypasses the duodenum and first part of the jejunum [3]. Therefore, this device limits the absorption of nutrients from the duodenum and proximal jejunum. EndoBarrier® (GI Dynamics, Lexington, MA, USA) was developed for this purpose (Fig. 1).
The duodenal-jejunal bypass sleeve is delivered endoscopically and fluoroscopically with a catheter-based system. The delivery system is introduced into the duodenal bulb over the guide wire and deployed to the jejunum. Once the sleeve is completely deployed, the anchor, a self-expandable nitinol stent, is positioned to form a capsule in the duodenal bulb, allowing the tips to attach to the gastrointestinal (GI) tract to decrease the risk of migration. The sleeve allows food to pass while preventing contact with the duodenum, and biliary and pancreatic secretions. After placement, the sleeve is maintained for 12 weeks. This can be removed endoscopically.
Weight loss occurs because the bypass in the duodenum and proximal jejunum prevents the mixing of digestive enzymes, bile, and undigested foods. These then mix together at the end of the duodenal-jejunal bypass sleeve. Accelerated delivery of chyme into the distal GI tract enables bypass of dysfunctional digestive and absorptive processes.
The first prospective, open-label, single-center trial was reported in 2008 [4], in which sleeves were successfully placed in 12 patients and maintained for 12 weeks. Early removal was reported due to abdominal pain in two patients. Esophageal and oropharyngeal mucosal tears were also observed during the sleeve removal, but were not significant. The mean percentage of excess weight loss (%EWL) at 12 weeks was 23.6%. Notably, 3 of 4 diabetic patients could stop medications, with normal fasting blood glucose levels only 24 hours after implantation. Another 12-week, prospective, randomized trial was reported [5]. In this study, 25 patients underwent sleeve placement, and 14 were treated with diet control alone; the sleeve could be maintained in 80% of the patients for 12 weeks. Major adverse events included 3 cases of upper GI bleeding, 1 anchor migration, and 1 stent obstruction. The %EWL at 12 weeks was 22% for the device, which was significantly higher than the 5% in controls. The first multicenter, randomized clinical trial was reported in 2010 [6]. Thirty patients received a duodenal-jejunal bypass sleeve, and 11 were in a diet control group. Technical success was observed in 26 of 30 patients. After initial placement, 4 devices were explanted prior to the end-point, because of migration, dislocation, obstruction, or continuous epigastric pain. Mean %EWL was 19.0% for device patients after 3 months, which was significantly higher than the 6.9% in controls. At 12 months, serum biochemistry was improved from baseline: glycated hemoglobin (HbA1c) levels from 7.0% to 5.7%; triglyceride levels from 142.9 to 98 mg/dL; and high-density lipoprotein levels from 47.0 mg/dL to 57.5 mg/dL. The complications were minimal and treated conservatively. Another prospective, randomized clinical trial of duodenal-jejunal bypass sleeves also showed good outcomes [7]. This 12-week study analyzed 13 participants in the duodenal-jejunal bypass sleeve group and 24 in the sham-operated group. The primary outcome, %EWL, was 11.9% and 2.7% for the duodenal-jejunal bypass sleeve and sham groups, respectively. In the duodenal-jejunal bypass sleeve group, 62% achieved 10% or more %EWL compared with 17% of those in the sham group. However, only 8/21 (38%) enrolled patients completed the study, because of GI bleeding (n=3), abdominal pain (n=2), nausea and vomiting (n=2), or an unrelated preexisting illness (n=1).
Metabolic improvement was also shown in other studies [8,9]. In a 52-week prospective, open-label clinical trial, 22 obese patients with type 2 diabetes underwent placement of a duodenal-jejunal bypass sleeve for 1 year [8]. Of these, 13 completed the 52-week study. Early removal occurred for the following reasons: device migration (n=3), GI bleeding (n=1), abdominal pain (n=2), principal investigator request (n=2), and discovery of an unrelated malignancy (n=1). At the end of the study, substantial reduction in HbA1c (−2.1%±0.3%) was observed. Another multicenter randomized controlled trial compared the efficacy of 6 months of duodenal-jejunal bypass sleeve placement (n=38) and dietary intervention (n=39) for obesity and type 2 diabetes [9]. The %EWL after 6 months was 32.0% in the duodenal-jejunal bypass sleeve group and 16.4% in the control group. HbA1c levels improved to 7.0% in the duodenal-jejunal bypass sleeve group and 7.9% in the control group. At 6 months after the trial, the %EWL of the duodenal-jejunal bypass sleeve group was 19.8%, versus 11.7% in the control group. HbA1c was 7.3% versus 8.0% for duodenal-jejunal bypass sleeve users versus controls, respectively.
Studies are summarized in the Table 1. The duodenal-jejunal bypass sleeve is safe and effective for weight loss and hyperglycemia. However, the weight loss obtained by clinical trials of the duodenal-jejunal bypass sleeve is disappointing. Although this device may not be considered a primary procedure for obesity treatment, duodenal-jejunal bypass sleeve trials showed excellent glycemic control in most patients and diabetes resolution in some patients. The EndoBarrier® was approved in Europe and is indicated to treat patients with type 2 diabetes and obesity for 12 months. Device migration continues to be the most important safety concern. It is undergoing clinical trials in the USA.
Gastroduodenal-jejunal bypass sleeve
This device is theoretically similar to the EndoBarrier®. However, its sleeve is anchored at the esophagogastric junction and extends through the stomach about 120 cm into the small bowel, mimicking the final anatomical structure in Roux-en-Y gastric bypass surgery (Fig. 2A). As a result, food directly passes from the esophagus to the intestine and nutrient absorption is impossible throughout the stomach, duodenum, and jejunum. An Use of an endoscopically implantable and removable gastroduodenojejunal bypass sleeve (ValentTx Endo Bypass System, Inc., Hopkins, MN, USA) was first reported in 2011 (Fig. 2B) [10]. In this study, 22 patients with a mean baseline body mass index (BMI) of 42 kg/m2 were treated with the device. Of these, 17 maintained the device (77%), completed the 12-week study period, and had 39.7% EWL. Glycemic control was effective during the implantation of the device. All diabetic patients (n=7) had normal glucose levels throughout the study period without medications.

(A) Schematic image of Roux-en-Y gastric bypass. (B) Illustration of gastroduodenojejunal bypass sleeve.
The same author reported the first series of patients (n=10; mean BMI, 42 kg/m2) with 1-year implantation of the gastroduodenal-jejunal bypass sleeve [11]. Of these, 6 had fully attached and functional devices throughout the follow-up, and their mean %EWL was 54%. In the remaining 4 patients, partial detachment was found at follow-up endoscopy and mean %EWL was lower than in the fully attached group. Comorbidities including diabetes, hypertension, and hyperlipidemia were improved during the 1-year trial. Although initial experience with endoluminal gastroduodenojejunal sleeve is promising, further studies are needed.
Studies are summarized in the Table 1.
CONCLUSIONS
Results of several studies of malabsorptive endoscopic procedure on animals and humans have been published, in which this method led to weight reduction. This procedure can also offer treatment for obesity-related comorbidities, even though the efficacy was not fully satisfactory for weight loss, and the safety issues should be addressed. Improvement in technique will follow the development of new devices.
Notes
Conflicts of Interest: The author has no financial conflicts of interest.