Quality Indicators for Small Bowel Capsule Endoscopy
Article information
Abstract
Capsule endoscopy (CE) enables evaluation of the entire mucosal surface of the small bowel (SB), which is one of the most important steps for evaluating obscure gastrointestinal bleeding. Although the diagnostic yield of SB CE depends on many clinical factors, there are no reports on quality indicators. Thus, the Korean Gut Image Study Group (KGISG) publishes an article titled, “Quality Indicators for Small Bowel Capsule Endoscopy” under approval from the Korean Society of Gastrointestinal Endoscopy (KSGE). Herein, we initially identified process quality indicators, while the structural and outcome indicators are reserved until sufficient clinical data are accumulated. We believe that outcomes of SB CE can be improved by trying to meet our proposed quality indicators.
INTRODUCTION
Capsule endoscopy (CE) was introduced in 2000 [1] and since then, it has become one of the first-line diagnostic tools for small bowel (SB) disorders [2]. Currently more than 4000 studies addressing CE can be found using PubMed. Additionally, applications of CE have been expanded to the evaluation of esophageal and colorectal disorders.
CE is the best method to evaluate the entire mucosal surface of the SB and it plays a key role in evaluation of obscure gastrointestinal bleeding (OGIB). However, the diagnostic yield of SB CE can be affected by many factors, such as indications, bowel preparation, technical errors, view mode and frame rate during interpretation, reviewers’ experience, and so on.
Diagnostic procedures with inadequate quality may be related with decreased diagnostic accuracy, procedure-related complications, and unnecessary medical burden. However, there are no publications on quality indicators for SB CE. Thus, the members of Korean Gut Image Study Group (KGISG) decided to establish “Quality Indicators for Small Bowel Capsule Endoscopy” under approval from the Korean Society of Gastrointestinal Endoscopy (KSGE).
Generally, quality indicators refer to specific issues identified for comparison and potential improvement [3] and they represent a minimally acceptable standard of care [4]. Quality indicators are classified as structural, process and outcome measures based on the type of assessment. Structural indicators evaluate the health care environment and they are typically scrutinized during accreditation surveys. Process indicators represent care delivery performance and outcome indicators suggest results of care [3]. Herein we describe process indicators. Structural and outcome indicators have not been covered in this article since there are insufficient clinical studies and evidences regarding the standards of care environments and minimal requirements for care results.
We believe the following quality indicators will be helpful to establish competence of CE and to improve quality of CE.
METHODS
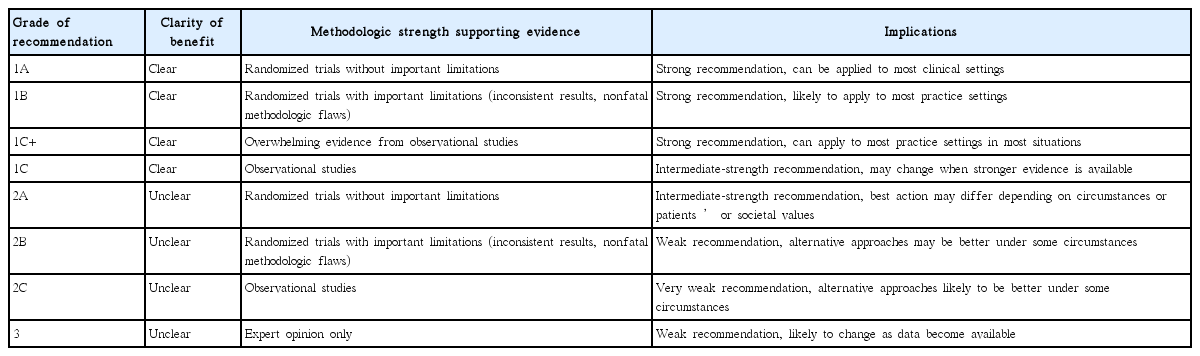
Three taskforce teams (preprocedure, intraprocedure, and postprocedure) from KGISG were formed and each team performed systematic literature search and prepared a comprehensive review to identify articles relevant to CE since the year 2000, using PubMed, MEDLINE, KoreaMed, and Google Scholar. Each team generated indicator candidates. Finally, 16 quality indicators were selected after repetitive discussions. Each candidate indicator, proposed by a team, was reviewed and discussed by other teams in a crossover manner. The grades of recommendation were classified in Table 1 based on levels of evidence [5,6]. The proposed preprocedural, intraprocedural, and postprocedural quality indicators common to SB CE are listed in Table 2.
PREPROCEDURAL QUALITY INDICATORS
Indications
OGIB
Capsule endoscopy is recommended as the first-line investigation for patients with obscure gastrointestinal bleeding (grade of recommendation: 1A).
The overall diagnostic yield of CE for OGIB ranges between 30% and 70%, which is higher than that of other diagnostic modalities (push enteroscopy, double-balloon enteroscopy [DBE], and SB series with sensitivities of 31%, 23%, and 5%, respectively) [7-9]. CE should be performed as soon as possible after the bleeding episode, optimally within 14 days, to maximize the diagnostic yield [7]. The causative lesions include intestinal angiodysplasia, SB ulcer, blood in the SB without an identified lesion, SB tumors, and SB varices [9,10]. From about a half to two thirds of patients receiving non-steroidal anti-inflammatory drugs (NSAIDs) have SB lesions such as erosions, petechiae, denudated mucosa, bleeding lesions, ulcers, and etc [11]. The role of CE is clearer in patients with OGIB after negative results of conventional endoscopy. Iron deficiency anemia (IDA) is usually determined based on blood loss through the gastrointestinal (GI) tract. Therefore, CE is a good method to identify causative lesions once other common potential bleeding sources located within the reach of conventional endoscopies have been excluded. Additionally, CE is useful in determining the route of device-assisted enteroscopy (DAE).
Crohn’s disease
Capsule endoscopy is the most sensitive diagnostic modality for detecting mucosal lesions of the small bowel in patients with suspected or established Crohn’s disease (grade of recommendation: 1B).
CE plays a role in diagnosing suspected Crohn’s disease (CD) when the clinical history is compatible, but not as much as diagnostic ileocolonoscopy. SB endoscopy helps to make differential diagnosis of CD in suspected CD patients by identifying SB involvement proximal to the terminal ileum. It also plays a role in the mucosal severity of the SB and disease extension in patients with established CD [7,12-14]. Meta-analysis showed that the diagnostic yield for SB CD was higher (50%–70%) with CE than with other diagnostic modalities (SB series, 22%; colonoscopy, 48%; push enteroscopy, 8%; and enteroclysis/computed tomography enterography [CTE], 31%) [12]. When stenosis or obstruction is suspected, patency capsule or careful examination using cross-sectional imaging modalities, such as magnetic resonance (MR)/CT, should be considered first.
SB tumor and polyposis syndrome
Capsule endoscopy is useful for detection of small bowel tumors and polyps (grade of recommendation: 2C).
CE is recommended for patients with OGIB to find SB tumor. However, DAE is preferred rather than CE in patients with SB tumor-suspected images [7]. The most common presentation of SB tumors is OGIB and the most common histopathological type of these tumors is adenocarcinoma, followed by carcinoid tumors, lymphoma, sarcoma, and hamartoma. The most common location is the jejunum (40%–60%), followed by the ileum (25%–40%) and duodenum (15%–25%) [10]. CE is also useful in detection of SB polyps and surveillance in familial adenomatous polyposis and Peutz-Jeghers syndrome [7,15].
Celiac disease
The use of CE for suspected celiac disease is not routinely recommended. However, it is suggested to use CE in patients unwilling or unable to undergo conventional endoscopy [7].
Abdominal pain of unknown origin
CE has a low diagnostic yield in patients with abdominal pain (13%); however, the diagnostic yield of CE can be improved in patients with chronic abdominal pain accompanied with elevated serum inflammatory markers (C-reactive protein, erythrocyte sedimentation rate), weight loss, diarrhea, and so on [16].
Identifying high-risk patients for capsule retention
In patients with Crohn’s disease, previous abdominal surgery, intestinal ischemia, volvulus, and history of abdominal radiotherapy, the risk of capsule retention is increased. For risk stratification and prevention of capsule retention, taking careful clinical history and performing careful physical examination is essential (grade of recommendation: 3).
The capsule is usually excreted with feces within 24–48 hours of ingestion. Capsule retention (CR) is defined as having a capsule remain in the GI tract for more than 2 weeks or requiring directed surgical or endoscopic intervention [17]. The known rate of CR is almost 0% in healthy adults, 13% in patients with suspected or known CD, and 16% in patients with symptomatic SB obstruction [18].
In cases of CD, up to 66% of patients have SB involvement at diagnosis. Twenty-five percent of patients with CD have had at least one SB stricture [19]. Data from the hospital-based study with 16-year follow-up period showed that the stricture occurred in 20.1% of CD cases [20]. Other known risk factors associated with CR are NSAIDs enteropathy, previous abdominal surgery, intestinal ischemia, volvulus, and history of abdominal radiotherapy [17].
In subjects with Crohn’s disease, obstructive symptoms, and suspicious stenosis, small bowel imaging, such as computed tomography enterography or magnetic resonance enterography, should be methods of choice for patency of small bowel prior to subsequent capsule endoscopy. Additionally, the use of patency capsule to confirm the functional patency of small bowel is recommended (grade of recommendation: 2C).
Since CTE/CT enteroclysis or MR enterography (MRE)/MR enteroclysis could identify strictures and assess the transmural or extraluminal nature of the disease, if subjects have obstructive symptoms or suspicious stenosis, dedicated SB cross-sectional imaging with CTE or MRE generally takes precedence over CE for evaluation of the SB [7,21]. In CD, the findings of SB stenosis at CTE or MRE may preclude prior to CE in 27% to 40% [22]. In the studies using patency capsule instead of CE, MRE has a high negative predictive values (96.3%–100%) and sensitivity (92.3%–100%) for patency CR [23].
Patency capsule is used before CE to evaluate the patency of the GI tract in patients with stricture or suspected stricture [17]. According to a study comparing CR rates in high risk patients evaluated with patency capsule and SB cross-sectional imaging, it was more predictable for high risk patients with negative patency capsule to have negative CR than for those who received SB cross-sectional imaging [24]. CE following positive patency capsule in patients with CD was associated with a high risk of CR. However, the risk of CR was not reduced by nonselective use of the patency capsule [25]. Recently, novel radio frequency identification (RFID) tag-less patency capsule has been introduced to clinical practice to reduce associated risks of the typical patency capsule. The impact of the patency capsule’s inner RFID tag in a stricture could cause SB ileus. Use of the tag-less patency capsule can confirm GI tract patency in most of the patients who did not have stenosis during imaging and allowed estimation of the patency for patients who did have stenosis on imaging [26].
Patient instructions
Intraprocedural and postprocedural patient instructions should be provided in written form before performing small bowel capsule endoscopy (grade of recommendation: 3).
Little is known about whether the diagnostic yield or quality of CE can be influenced by the intraprocedural and postprocedural patient instructions. In general, the manufacturer’s guidelines include the acceptable physical activity and dietary intake after swallowing the capsule to ensure successful tests [27,28]. Early clinical studies related to CE also specifically described the instructions to the patients after capsule ingestion [29,30]. These instructions mainly include recommendations related to physical activity and acceptable dietary intake while tests are proceeding. In addition, patients should be instructed about when they should return data recorders or suspect the possibility of CR after completion of tests. Details of general instructions are summarized in Supplementary Tables 1 and 2. If needed, individualized instructions could be provided or ordered by the physicians. For example, dietary intake should be restricted for the patient with ongoing overt OGIB who may require urgent or emergent intervention based on the results of CE.
INTRAPROCEDURAL QUALITY INDICATORS
Bowel preparation
Quality of adequate bowel preparation
Excellent or good preparation (>75% small bowel visualization) is considered to enhance diagnostic yield of small bowel examination (grade of recommendation: 1C).
During CE, several factors, such as bubbles, food material in the SB, and gastric and SB transit time, influence the SB visualization quality (SBVQ), diagnostic yield, and cecal completion rate were analyzed. Therefore, bowel preparation prior to CE is as important as bowel preparation prior to colonoscopy. Diagnostic yield is defined as a meaningful diagnostic finding of CE. Purgative bowel preparations enhance diagnostic yield of SB examination using CE [31].
SBVQ was defined as follows: “excellent,” if an ideal visualization of the SB mucosa was achieved (>90%); “good,” if >75% of the mucosa was in perfect condition; “fair,” if only 50%–75% of the mucosa was under perfect conditions; and “poor,” if <50% of the mucosa could be observed. However, there was no consensus of optimal bowel preparation for CE, as each study with polyethylene glycol (PEG) suggested various definitions for bowel preparation quality. A recent study considered excellent or good preparation (>75% SB visualization) as an adequate bowel preparation [32].
Purgatives of adequate bowel preparation
Bowel preparation with purgatives enhances the small bowel visual quality compared with fasting alone or a clear liquid diet (grade of recommendation: 1B).
To date, there have been many comparative studies, consensus, and guidelines regarding different types of bowel cleansing agents in bowel preparation for CE [33]. Currently, PEGbased regimens are primarily recommended. A 2-L PEG with simethicone is most frequently recommended for CE bowel preparation. Sodium picosulphate-based regimens are recommended as a second option, as their cleansing efficacy is less than that of PEG-based regimens.
According to the guidelines for bowel preparation, which were published by the KGISG in 2013 [34], bowel preparation with PEG compared with fasting alone or clear liquid diet enhances diagnostic yield and SBVQ without effect on cecal completion rate. A 2-L PEG solution for bowel preparation is similar to that of a 4-L PEG solution in diagnostic yield, SBVQ, and cecal completion rate of CE [35,36]. Bowel preparation by fasting or administration of PEG solution, when combined with simethicone, enhances SBVQ, but does not affect cecal completion rate of CE [37-40]. Bowel preparation using prokinetics does not enhance SBVQ, diagnostic yield, or cecal completion rate of CE [34]
Currently, there has been no consensus regarding optimal timing of bowel preparation before CE. A 2-L PEG-based purge, administered one day prior, is the most commonly used preparation method. In clinical practice, CE exam is followed by colonoscopy in patients with obscure GI bleeding or CD. Therefore, the timing of bowel preparation is frequently dependent on the time of colonoscopic examination. According to a single center study by Black et al., [41] there was no significant difference of diagnostic yield, and the quality according to timing of SB preparation (14 hours vs. 4 hours prior to the CE). The day before bowel preparation showed similar results to the same-day bowel preparation regarding quality, SB transit time, cecal completion rate, and overall diagnostic yield. Considering colonoscopic bowel preparation, shorter time interval between bowel preparation and CE may result in superior visualization. Further study is required for clarification.
Completion rate
Photodocumentation of capsule passing through the ileocecal valve or into the colon is necessary for verification of entire small bowel exploration (grade of recommendation: 2C).
Although CE is a useful tool for evaluating SB disease, it is impossible to visualize the entire SB in all patients because capsules have not always passed through the ileocecal valve before battery exhaustion due to various reasons [42].
Complete examination was defined as capsule passing through the ileocecal valve or into the colon on images during its working time [42] To increase diagnosis yield, complete examination rate of the entire SB is important.
The incomplete examination rate of the CE based on the 10-year data from the Korean CE registry was 33% (969/2,914) [43]. Multiple logistic regression analysis indicated that completion rates were significantly higher with better bowel preparation and in patients with OGIB. The incomplete rate was significantly higher in elderly patients.
Hospitalization, history of SB surgery, and delayed gastric emptying had been shown to be risk factors for incomplete examination [44]. Effort to increase complete examination rate is important in order to maximize the diagnostic yield of CE.
When capsule endoscopy is performed in patients with high risk of delayed gastric emptying, identifying capsule’s position using plain radiography or real-time viewer after ingestion, or endoscopic employment of capsule endoscopy is recommended (grade of recommendation: 1B).
Gastric retention resulting in capsule failing to enter the duodenum and delayed gastric transit, remaining in the stomach for more than 1–1.5 hours had been known as the major causes for incomplete examination [45].
The effects of routine use of prokinetics, such as metoclopramide and erythromycin, to enhance gastric transit were inconsistent [46-50]
Identifying capsule’s position using plain radiography or real-time viewer two hours after ingestion is recommended as the effective method to reduce gastric transit time, especially in patients who are more likely to have delayed gastric transit. Particularly, use of external real-time viewer to check the progress of the capsule and prespecified actions, such as additional water swallowing, administration of prokinetics, and endoscopic delivery, significantly improved the completion rate [51-53].
Although it is invasive, endoscopic delivery of capsule to the duodenum has been used as an optimal method to reduce gastric transit time. Additionally, it can be useful in patients with high risk of delayed gastric emptying, particularly in patients with diabetes, vagotomy, scleroderma, or ongoing hospitalization [53-55].
Technical errors
Technical errors during capsule endoscopy procedure can decrease quality of capsule endoscopy images, although, it seldom occurs (grade of recommendation: 3).
CE image is of significant importance, because high-quality images provide more information for diagnosis. It is not satisfactory mainly because of factors that reduce CE quality. Images obtained during CE are exposed to different types of noise; for example, food and gas in the stomach, SB, or colon can reduce image quality. Captured images tend to have technical errors [56].
Together, results of previous studies allow technical errors to be divided into two types: CE system-related error (communication and transmission error) and patient-related error. CE system-related errors include low resolution, blurred images, and light-related distortions. One of the typical noise types in the capsule image was due to data communication errors [57]. The CE image quality can be improved by removing noise using a median filter or a smart antenna technique, based on the results of these studies [58]. Patient-related technical errors in CE images can occur in patients with implantable cardiac devices (ICD). The presence of an ICD is one of the relative contraindications for CE. CE interference may arise during the procedure, resulting in alterations of the ICD [59]. However, few studies show actual interference between the ICD and CE [60]. Left ventricular assist devices (LVADs) had the tendency to interfere with CE image capture; therefore, a positioning the CE lead as far away from the LVAD as possible was required [61,62]. Recently, interference-related problems between CE and LVADs are expected to be solved by replacing the lead sensor with a sensor belt.
Hence, CE image quality plays a critical role in diagnosis. Although there are no published reports of the technical error rate in practice, it is generally considered to be very low. However, because technical errors during the CE procedure are associated with quality of the CE image, these need to be reported more carefully. Based on these results, action should be taken to solve or reduce technical errors for better quality CE images.
POSTPROCEDURAL QUALITY INDICATORS
Interpretation
View mode
DualView or QuadView may be recommended as the viewing mode to improve reading efficiency and detection rate of interpreters reading capsule endoscopy (grade of recommendation: 2C).
To date, there is no optimal view mode for the best interpretation of CE. However, the appropriate selection image number at the monitor improves reading efficiency and detection rate of interpreters reading CE [63]. Currently, softwares for CE reading have several view mode options, including SingleView, DualView, and QuadView, according to the number of images shown in one monitor [64]. In a survey including 530 members of the American College of Gastroenterology, SingleView, DualView, and QuadView were used by 23.7%, 40.4%, and 54.5%, respectively [65]. QuadView has a theoretical advantage of longer single frame exposure time compared to SingleView, helping interpreters in detecting more lesions [63,66] However, QuadView might have a drawback of making readers rely on their peripheral vision because of simultaneous focusing on several images [66].
A previous study evaluated the effect on the SB using 10 selected video clips. The playing time from the entry of the capsule into the duodenal bulb to the cecum, without the video being stopped once started, was determined by 11 different combinations of video mode and frame rates in order to evaluate the impact of view mode on reading time and detection rates of lesions. There was no difference in reading time according to view mode at the same frame rate [63]. However, detection rate was higher in DualView or QuadView than in SingleView: 56% in SingleView, 83% in DualView, and 85% in QuadView at 10 frames per second (f.p.s). There was no significant difference in detection rates between DualView and QuadView regardless of frame rates. Therefore, DualView or QuadView may be recommended for the selection of view mode during CE reading because of higher detection rates compared to SingleView. Because it has not been determined whether the appropriate view mode for the highest detection rate during CE reading is DualView or QuadView, interpreters can select one of these view modes based on their preference.
Frame rate
During capsule endoscopy reading, 15 frames per second or less is appropriate for acceptable detection rate (grade of recommendation: 2C).
The frame rate means the number of f.p.s that is displayed in a video file by softwares for CE reading. Currently, these softwares provide a various range of frame rates allowing reduction of reading time of thousands of capsule’s images [64]. In a study evaluating the effect of frame rate on reading time and detection rate, slower frame rate increased reading time irrespective of view mode options: compared to 10 f.p.s, the mean playing time of videos shortened by 33%, 61%, and 72% in 15, 25, and 40 f.p.s, respectively [63]. In contrast, when interpreters were asked to manually count angioectasia of each positive image from video clips, detection rates calculated using maximum number of positive images were 75% in 10 f.p.s, 51% in 15 f.p.s, and 36% in 25 f.p.s. There was a significant difference in detection rates according to frame rates. In a study evaluating detection rates among different options based on view mode and frame rates, detection rates were 45% in SingleView 15 f.p.s, 26% in SingleView 25 f.p.s, 47% in QuadView 20 f.p.s, and 43% in QuadView 30 f.p.s [66]. These studies suggest that slower frame rates can result in higher detection rates during CE reading despite the need for longer reading time.
According to a panel consensus of 2002 International Conference of Capsule Endoscopy, the fastest acceptable frame rate of review was 15 f.p.s [67]. However, the consensus was made by experienced interpreters in CE reading and the frame rate option could have been determined based on their own experience. Actually, a previous study suggested that trainees should begin using the frame rates of 5–10 f.p.s for optimal interpretation [68]. Based on these results, 15 f.p.s or less (combined with DualView or QuadView) is recommended for appropriate frame rate during CE reading. Although, the optimal frame rate for acceptable detection rate and reading time is unclear. Further studies are needed to determine the best option for appropriate interpretation based on view mode and frame rate in real practice, considering the experience of interpreters and the optimal lower limit of detection rates.
Reviewer
Experience with minimum of 10–20 capsule endoscopy cases is required for trainees to attain capsule endoscopy competency (grade of recommendation: 1C). Because the lesion miss rate during capsule endoscopy is relative high, interpretation of findings in capsule endoscopy should be done by experienced and competent endoscopists. Interpretation performed by a trainee should be reviewed and confirmed by an expert (grade of recommendation: 2C).
The utility of CE as a diagnostic tool depends on the accuracy of interpretation. However, lesion miss rate is high possibly because only a small fraction of images shows clinically significant lesions during the reading of capsule images [66]. The miss rate in CE reading was reported to be 11% of all SB findings and 18.9% for single-mass lesions [69]. The American Society of Gastrointestinal Endoscopy recommended that interpretation of CE should be performed by endoscopists who completed a GI endoscopy training program for the diagnosis and management of small intestinal disease [70]. However, there is no data addressing issues regarding standardized competency assessment tools and minimum training requirements to ensure competence for CE. Although published guidelines including American Gastroenterological Association, American Society of Gastrointestinal Endoscopy, and KSGE have recommended 25, 20, and 10 cases of CE, respectively as necessary to ensure competence in the interpretation of findings [70-72], these recommendations are made based mainly on societal guidelines and expert opinions. Recently, two prospective studies have investigated issues regarding CE learning curves [73,74] One single-center prospective study investigated 39 GI fellows based on structured CE training curriculum [73]. In this study, fellows were grouped according to the number of completed CE interpretations. Mean scores for trainees with fewer than 10, 11 to 20, and 21 to 35 CE interpretations were 79%, 79%, and 85%, respectively. A significant difference was seen between staff and fellow scores with 10 or fewer and 11 to 20 interpretations, respectively (p<0.001). Furthermore, no correlation was observed between CE test scores of trainee and previous endoscopy experience. Another Korean multicenter study also evaluated the number of cases needed for trainees to gain necessary experience for CE competency [74]. Most of the mean kappa coefficients were >0.60 and >0.80 after week 9 and 11, respectively, which indicates a good agreement of the trainees with the expert after 9 weeks and a very good agreement after 11 weeks. In this study, approximately 10 cases of CE were suggested as a minimum number of CE required for trainees to attain competency. Taken together, learning curve of CE interpretation seems to include 10–20 cases of CE for a trainee who has competency in wired endoscopy. During this period, continuous guidance and feedback under proper supervision is warranted.
However, the effect of interpreter’s experience on detection of lesions during CE reading has not been fully determined. A recent study including 17 endoscopists with experience from 23 to over 1,000 total CE procedures showed that experience of capsule reading did not significantly affect detection rate: 17% of the interpreters with the lowest detection rates had reading experience of more than 300 capsules [66]. Although we cannot confirm the effect of experience on detection of the lesion, the inter-observer variability in CE reading is different according to the amount of experience. Several studies showed that the inter-observer variability was higher among non-experienced endoscopists [75-77]. There has been no study about the additional benefit of interpretation by two experts; however, in a case of a trainee, a review made by an expert is essential for the improvement of interpretation quality.
Complications
Either conservative or endoscopic treatment can be considered for capsule retention, and the decision depends on patient’s symptoms or availability of enteroscopy. Surgical removal of retained capsule could be reserved for asymptomatic patients (grade of recommendation: 3).
CE has been proved to be an effective and safe device in the diagnosis of SB disease with few complications or adverse effects. Retention of capsule, perforation, aspiration, and SB obstruction are reported as complications of CE. Among these, CR is the most common complication. Retained capsules are usually asymptomatic but may cause partial or complete intestinal SB obstruction in some patients. Several cases where a CR leads to intestinal perforation have been reported [78,79]. A systemic review by Liao et al. stated that among the 164 retained capsules reported in 122 articles, most were surgically removed (58.7%, 108/164) [42] However, according to the full texts of 128 articles, of the 104 retained capsules with clinical symptoms mentioned, 88 were asymptomatic and only 16 were associated with partial or complete intestinal obstruction [42]. In the case series presented by Höög et al., surgical removal was performed in 27 out of 31 patients with CR [80] However, only 9 of 27 patients showed obstructive symptoms. Moreover, there were postoperative deaths in 3 of the 27 cases, 2 due to anastomotic breakdown and 1 due to multi-organ failure [80]. Considering post-operative complications, decision of surgical removal should be made very carefully. Recent studies showed more favorable results with conservative management, such as treating the underlying disease, in patients with CD, with corticosteroids or anti-tumor necrosis factor (TNF) antibodies, and cessation of NSAIDs in patients with drug-induced enteropathy. Such conservative management induced spontaneous passage of the capsule in 52.4% of cases [81]. The longest duration of a case with CR was 6 years and 10 months, which had been asymptomatic. During follow up period, there was also no breakdown of CE. In the case series by Höög et al., one patient who received anti-TNF therapy showed CR due to a CD [80]. He then expelled capsule 2.5 years later [80,82]. Such cases suggest that conservative or medical management may be a good option in patients with asymptomatic CR. However, a case of disruption of retained CE after 3 years was also reported. Therefore, a possibility of CE disruption should be informed during “wait and see” strategy period. Another option is endoscopic removal using DBE or single-balloon enteroscopy. Recent study reported treatment strategy for CR by DBE. Their result revealed that 72.7% (32/44) of retained CE could be successfully retrieved by enteroscopy [83]. Among 12 cases with unsuccessful removal by enteroscopy, spontaneous passage of retained capsule after medication therapy was observed in 11.4% (5/44) of patients, only 6.8% (3/44) needed surgery. Abovementioned results suggested the feasibility of enteroscopic removal of retained capsule and clarified the factors associated with successful removal (jejunal location, antegrade insertion, three or fewer strictures). Abovementioned results also showed successful conservative and endoscopic management of CR. Based on these reports, if there are no symptoms associated with CR, conservative or endoscopic treatment can be considered, and surgical removal of retained capsule could be reserved.
CE report
Procedure reports are required for every capsule endoscopy and should be accurate, concise, and completed in a timely manner (grade of recommendation: 3).
Accurate and timely documentation of capsule endoscopic findings and recommendations improve patients care. The task force emphasizes that the CE report should be detailed. The patient’s medical history justifying the CE should be described. The indications are classified into signs and symptoms, and diseases. Standardization of the language and structure of endoscopic reports based on CE structured terminology (CEST) may improve communication between clinicians [84]. To define the location of lesions more precisely, the temporal location should be defined by time points recorded by CE. The following locations can be detected by specific time-point of landmarks: time of ingestion, time of the first gastric image, time of the first duodenal image, and time of the first colonic image. For practical description, diagnoses are divided into main diagnoses and other diagnoses, which are less important or less associated with the reason for CE. Complications are described as unexpected events that occur during or after the CE. Minimal elements of a CE report are summarized in Table 3 [72,84,85].
CONCLUSIONS
We expect that the outcome of SB CE can be improved by trying to meet the abovementioned 16 quality indicators. Improvement of the outcomes should be confirmed by following studies. Continuous process of quality improvement should be followed.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
Supplementary materials are available at https://doi.org/10.5946/ce.2017.030 or via http://e-ce.org/.