Endoscopic Palliation for Biliary and Pancreatic Malignancies: Recent Advances
Article information
Abstract
Malignancies of the pancreatobiliary system are usually unresectable at the time of diagnosis. As a consequence, a majority of these cases are candidates for palliative care. With advances in chemotherapeutic agents and multidisciplinary care, the survival rate in pancreatobiliary malignancies has improved. Therefore, there is a need to provide an effective and long-lasting palliative care for these patients. Endoscopic palliation is preferred to surgery as the former is associated with equal efficacy and reduced morbidity. The main role of endoscopic palliation in the vast majority of pancreatobiliary malignancies includes biliary and enteral stenting for malignant obstructive jaundice and gastric outlet obstruction, respectively. Recent advances in endoscopic palliation appear promising in imparting long-lasting relief of symptoms. Use of radiofrequency ablation and photodynamic therapy in malignant biliary obstruction has been shown to improve the survival rates as well as the patency of biliary stents. The emergence of endoscopic ultrasound (EUS) as a therapeutic tool has enhanced the capability of minimally invasive palliation in pancreatobiliary cancers. EUS is a valuable alternative to endoscopic retrograde cholangiopancreatography for the palliation of obstructive jaundice. More recently, EUS is emerging as an effective primary modality for biliary and gastric bypass.
INTRODUCTION
Malignancies of the pancreas and biliary system are among the leading causes of gastrointestinal cancer-related mortality. The majority of pancreatobiliary malignancies are unresectable at the time of presentation. Therefore, palliation of symptoms including pain, jaundice, and pruritus is the main objective in these patients. Endoscopic palliation in these patients is minimally invasive and therefore associated with decreased morbidity compared to palliative surgery. With recent advances in systemic chemotherapy, survival rates have improved especially in pancreatic cancer [1]. This means that palliation of symptoms should not only be effective, but also long-lasting to avoid morbidity-related repeated interventions. Endoscopic palliation in pancreatobiliary malignancies include biliary drainage, enteral stenting for gastric outlet obstruction, and celiac plexus neurolysis for relief of pain.
Over the last decade, the role of endoscopy as a palliative modality has broadened with the availability of novel tools and techniques. The development of endoscopic ultrasound (EUS) as a therapeutic modality has allowed a wide array of remedial procedures to be performed endoscopically.
ENDOSCOPIC PALLIATION OF MALIGNANT BILIARY OBSTRUCTION
A large proportion of cases with malignant biliary obstruction (MBO) are unresectable at the time of presentation. The aim of palliation in these patients is to improve the quality of life by reducing jaundice and pruritus. Endoscopic palliation is preferred over surgery as the former is associated with reduced morbidity and equal benefits. Self-expandable metal stents (SEMS) are preferred over plastic stents for endoscopic palliation of patients with MBO. The use of SEMS results in longer stent patency, lower complication rates, fewer re-interventions, and possibly increased survival rates [2]. In addition, SEMS placement results in better scores for general and disease-specific health related quality of life compared with plastic stent placement [3]. Although SEMS are more expensive than plastic stents, there is no significant cost difference in the long term due to reduced requirements for re-interventions [4]. The choice of SEMS (covered or uncovered) is largely operator-dependent as there is no significant difference with regard to the clinical outcomes [5]. Covered SEMS have lower tissue ingrowth, but higher migration rates and tissue overgrowth as compared to uncovered SEMS.
The main issue with biliary drainage using stents is re-occlusion. In order to prolong stent patency, various strategies have been evaluated including incorporation of chemotherapeutic agents into the covering material of stents and ablating the tumor using radiofrequency ablation (RFA) prior to stent deployment. Drug-eluting stents were evaluated in animal and small cohort human studies with the hope of reducing stent dysfunction due to tumor ingrowth [6-10]. Although there were no major safety issues, the outcomes were sub-optimal with regard to the improvement in stent patency. More recently, a vorinostat-eluting poly (DL-lactide-co-glycolide) nanofiber-coated stent was evaluated for the inhibition of cholangiocarcinoma cells. Vorinostat is a histone deacetylase inhibitor with inhibitory effects on growth and differentiation of malignant cells. Vorinostat-eluting nanofiber membranes showed significant antitumor activity against cholangiocarcinoma cells in vitro and in vivo [11]. Large, prospective trials are required to evaluate the clinical utility of novel drug-eluting stents in patients with MBO.
ENDOSCOPIC ULTRASOUND GUIDED BILIARY DRAINAGE
EUS has emerged as an immensely useful therapeutic modality for palliation in pancreatobiliary malignancies. Combined biliary and gastric outlet obstruction is not uncommon in advanced biliary and pancreatic neoplasms. In these cases, papilla may not be accessible and therefore endoscopic palliation of biliary obstruction with endoscopic retrograde cholangiopancreatography (ERCP) is often not feasible. On the other hand, biliary cannulation is occasionally unsuccessful due to neoplastic infiltration of the papilla or surgically-altered anatomy. Palliation of jaundice can be successfully accomplished using EUS guided approaches in such cases. When compared to percutaneous drainage, EUS-guided biliary drainage (EUS-BD) is equally effective, but associated with a lower rate of adverse events and fewer re-interventions [12,13]. Until recently, EUS-BD was used as a rescue option in patients with failed ERCPs. Experts in EUS have challenged this approach and utilized EUS-BD as a primary method for biliary drainage. Three randomized trials have compared EUS with ERCP as a primary modality for biliary drainage in cases with MBO (Table 1) [14-16]. Clinical outcomes in terms of clinical success and adverse events were equal in two trials [14,16], whereas EUS-BD was found to be superior to ERCP with longer stent patency, lower adverse events, and fewer re-interventions in one of the randomized trials [15]. With the development of dedicated devices and accessories, EUS-BD is likely to become a useful alternative to ERCP in MBO.
ENDOSCOPIC ABLATION IN PANCREATOBILIARY NEOPLASMS
Malignant biliary obstruction
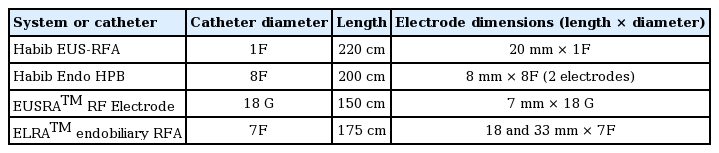
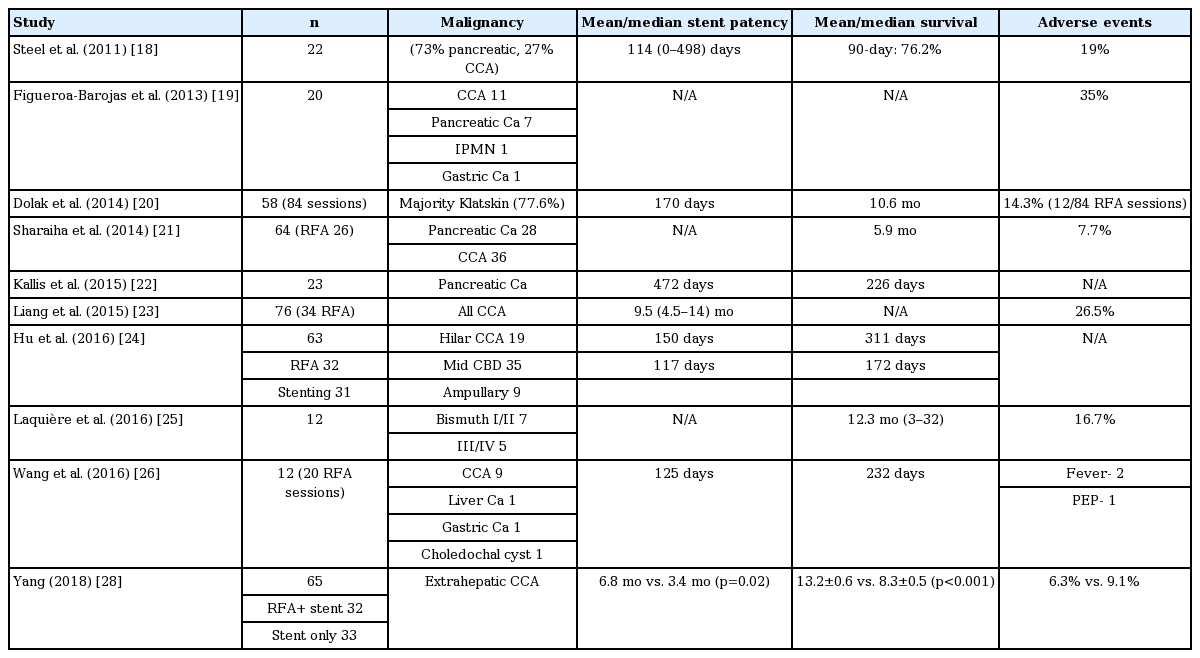
RFA and photodynamic therapy (PDT) are the main palliative modalities for unresectable cholangiocarcinoma. RFA is a thermal ablative tool based on the principle that heat causes coagulative necrosis and reduction in tumor volume. Recently, RFA is being increasingly utilized for the palliation of unresectable pancreatobiliary malignancies (Table 2) [17]. Steel et al., reported the first human application of RFA in 21 cases with MBO [18]. At 90 days, stent patency was documented in 16 of 21 patients [18]. Since this seminal study, the body of evidence is growing for the utility of RFA in MBO where it has been shown to prolong the patency of SEMS as well as improve the survival rates (Table 3) [18-28]. In a randomized study by Yang and colleagues, 65 patients with extrahepatic cholangiocarcinoma were randomized into stent only and RFA+ stent groups [28]. The mean stent patency period of the RFA+ stent group was significantly longer than that of the stent-only group (6.8 months vs. 3.4 months, p=0.02) [28]. In addition to improved stent patency, a few studies have also documented a survival benefit with adjunctive RFA [21,22,28]. In a randomized trial, the mean survival was significantly longer in the RFA+ stent group than in the stent-only group (13.2 +/– 0.6 months vs. 8.3 +/– 0.5 months, p<0.001) [28]. In a systematic review and metanalysis including nine studies (505 patients), the pooled weighted mean difference in the stent patency was 50.6 days in favor of RFA. Overall survival was also better in patients treated with RFA (hazard ratio, 1.395; 95% confidence interval, 1.145–1.7; p<0.001) [27].
The possible actions of RFA include opening up biliary strictures by debulking and preventing the proximal spread of tumor. In a recent study, the mean stricture diameter was 1.7 mm and 5.2 mm before and after endoscopic RFA, respectively [19]. In addition to local ablation, RFA also incites an immune response towards malignant cells and could be the possible reason for improved survival in these patients [27].
Adverse events with biliary RFA include abdominal pain, cholangitis, hemobilia, and cholecystitis. In addition, serious adverse events like partial liver infarction due to vascular injury have been reported after RFA [20]. The proximity of the proximal biliary tract to the hepatic artery may be responsible for such vascular events. It is important to note that some of these adverse events may not be related to RFA per se. For example, cholecystitis after SEMS placement, with or without preceding RFA, is mainly attributable to tumor involvement of the cystic duct. In a systematic review, abdominal pain was higher in the RFA group (31% vs. 20%, p=0.003). However, there was no significant difference between the RFA and stent-only groups with regard to cholangitis, acute cholecystitis, pancreatitis, and hemobilia [27].
The other palliative modality for cholangiocarcinoma with promising results in recent studies is PDT [29-32]. In an early randomized trial by Ortner and colleagues, PDT resulted in better biliary drainage and improved survival in patients with non-resectable cholangiocarcinoma (493 days vs. 98 days, p<0.0001) [32]. In a systematic review and meta-analysis, PDT was superior to biliary stenting alone with regard to successful biliary drainage (>50% reduction in bilirubin at day 7) and survival [33]. In contrast to the favorable outcomes in a majority of the studies, a recent randomized trial concluded that overall survival was worse in the PDT group as compared to the stent-alone group [34]. The results of this study should be interpreted with caution as there was a difference in the chemotherapy regimens in the two groups.
In a retrospective comparative study by Strand and colleagues, survival rates were similar between RFA and PDT (9.6 months vs. 7.5 months) [35]. However, a randomized trial is required to compare both these modalities. PDT is safe and major adverse events are uncommon. Self-limiting photosensitivity reactions can be seen in about 10% of the patients [33].
Pancreatic neoplasms
Endoscopic ablation has also been successfully used for the palliation of solid/cystic pancreatic neoplasms and for treating intraductal extension of ampullary neoplasms [36-42]. EUS-guided ablation was initially reported for cystic neoplasms of the pancreas in multiple small studies. Absolute alcohol (80%) as well as chemotherapeutic agents like gemcitabine and paclitaxel have been used as ablative agents in various studies [43,44]. One large study involving 164 cases, evaluated the long-term outcomes of endoscopic ablation in patients with pancreatic cysts [44]. A majority of these cystic lesions were either mucinous cystic neoplasms (43%) or indeterminate cysts (38%). At a median follow-up of 72 months, complete or partial resolution of cysts was noticed in 72.2% and 19.6% of cases, respectively. Of those with complete resolution, recurrence was found in only 2 patients (1.7%) [44]. Encouraging results of EUS-guided ablation in cystic neoplasms propelled the evaluation of endoscopic ablation in solid pancreatic tumors including neuroendocrine tumors and unresectable adenocarcinomas [38,41,42,45].
A majority of the patients with pancreatic cancer have locally advanced or metastatic disease at the time of presentation. The survival rate is poor in these cases and chemoradiotherapy provides only marginal benefits. Therefore, there is an unmet need for palliative modalities in this subgroup of patients. Intra-operative RFA has been shown to improve survival rates in stage III and IV pancreatic cancers [46,47]. In one study, intra-operative RFA in combination with palliative surgery resulted in significantly better survival rates than palliative surgery alone in patients with advanced pancreatic cancers [46]. In contrast to intra-operative RFA, EUS is minimally invasive and carries the ability to visualize and accurately target deep-seated pancreatic lesions in real time. Goldberg and colleagues demonstrated the feasibility and safety of EUS-RFA in thirteen Yorkshire pigs [48]. A 19 G needle was used for ablation which was insulated except for the distal 1 to 1.5 cm. For each ablation, RF was applied for 6 minutes and the electrode tip temperature of 90°±2° C was maintained. Hyperechoic foci measuring about 1 cm and representing the area of coagulation necrosis were observed after RFA. Complications included focal pancreatitis in one, intestinal burn in one, and gastric burns in three pigs [48]. Subsequently, several small cohort human studies have evaluated the technical feasibility and safety of EUS-RFA in pancreatic neoplasms (Table 4) [38,41,42]. In a small study including 6 patients with stage III and IV pancreatic cancers, EUS-RFA was technically feasible in all the cases without any major complications [38]. However, most of these studies are small and depict only the safety and feasibility of RFA in these patients. The actual clinical benefit in terms of improvement in survival rates remains to be seen. Adverse events reported with EUS-RFA include abdominal pain, acute pancreatitis, pancreatic fistulas, and portal vein thrombosis [45,47]. The risk of pancreatitis appears to be higher when lesions are located in the pancreatic head and in close proximity to the main pancreatic duct (<5 mm). Placement of a prophylactic pancreatic stent and keeping a safety margin (>5 mm from pancreatic duct) have been suggested as ways to reduce the risk of pancreatitis [45]. Another strategy to reduce complications includes decreasing the RFA temperature from 105° to 90°C [47]. Recently, new cryothermal probes, which are internally cooled with carbon dioxide, have been evaluated in animal studies [49,50]. The cryogenic gas produces effective cooling and therefore less collateral damage is expected. In addition, it augments the devitalization such that less power input (16 W) is needed compared with conventional RF ablation systems (30–60 W), to obtain the same ablation results [49]. The safety of these probes over conventional ablation probes and their application in humans remains to be demonstrated.
Besides RFA, the use of EUS-guided laser (Nd:YAG) ablation and PDT has been described for ablating pancreatic tissue [51-54]. Di Matteo and colleagues initially demonstrated the feasibility of pancreatic laser ablation in a couple of animal studies [52,53]. Subsequently, the same group evaluated the outcomes of laser ablation in nine patients with locally advanced pancreatic cancers (mean size 35.4 mm) that did not respond to chemoradiotherapy [51]. A 300-μm flexible fiber (Elesta s.r.l., Florence, Italy) preloaded onto a 22 G fine needle was used for laser ablation. The power settings were 2–4 W/400–1,200 J and the ablation time ranged from 200–600 seconds. The ablation was completed for all patients with an ablation area of 0.4–6.4 cm3. There were no major adverse events [51].
Endoscopic-RFA has also been successfully used for ampullary adenomas with intraductal extensions [37,39,40]. These patients are often managed using Whipple’s surgery which has a high morbidity rate. In a series of 14 patients with adenoma extension into the common bile duct, RFA was successfully performed in 13 patients. Of these, surveillance-intraductal biopsy specimens showed no neoplasm in 12 patients. The main adverse event was ductal stricture in 5 patients [39].
Others
Other applications of EUS in pancreatobiliary malignancies include control of pain, placement of fiducial markers for image-guided radiotherapy, and delivery of antitumor agents into the tumor.
Pain is often a troubling symptom in patients with pancreatic cancer. Celiac-plexus neurolysis is recommended, but the effect is short-lived. Recently, RFA has been utilized for ablation of celiac ganglia in these patients [55,56]. Bang and colleagues compared EUS-RFA to traditional celiac-plexus neurolysis in a randomized trial [55]. The Habib EUS-RFA catheter (EMcision Ltd., London, United Kingdom) and a 19 G needle were used for ablation in this study. A total of 2 to 4 RFA applications (90 seconds, 10 W) were used. EUS-RFA provided better pain relief without any difference in the rate of adverse events. Importantly, 21% of patients with persistent pain after celiac-plexus neurolysis could be successfully managed with RFA. The proposed advantages of EUS-RFA over celiac-plexus neurolysis using alcohol include a predictable area of necrosis and immediate symptom relief [55]. However, the data is limited and the ideal settings of RFA have yet to be determined in these patients.
Another emerging application of EUS in pancreatic neoplasms is the delivery of antitumor agents into the malignant tissue. Systemic chemotherapy is not very effective in pancreatic cancers partly due to the limited penetration of chemotherapeutic agents. Antitumor agents that have been evaluated for EUS-guided injection include gemcitabine, genetically-modified viruses (adenovirus: ONYX-015, TNFerade; herpes simplex virus: HF10), double-stranded RNA oligonucleotide (STNM01), double-stranded DNA plasmid (BC-819), dendritic cells, and cytoimplants [57-61]. Preliminary data indicate the feasibility of EUS-guided injection of these agents. However, large scale studies are required to establish the safety and efficacy of endoscopic injection of antitumor agents in patients with advanced pancreatic cancer.
EUS-guided placement of inert radiopaque markers, i.e., fiducial markers into the tumor tissue, has been evaluated in patients with locally advanced pancreatic cancers. These markers serve as a guide for subsequent stereotactic body radiotherapy. Fiducial markers are available as radiopaque spheres or coils. Traditional fiducials are thicker (0.8 mm diameter) than coiled fiducials (0.35 mm diameter) and offer better visualization [62]. However, a 19 G needle is required for their deployment. Coiled fiducial markers (Visicoil; IBA Dosimetry, Bartlett, TN, USA) are thinner and can be deployed with a 22 G needle which may have greater flexibility and access to the tumor [63]. Multiple studies have confirmed the safety and feasibility of EUS-guided placement of fiducial markers [62-65]. There are two main techniques for EUS-guided fiducial insertion. In one technique, once the needle is inserted into the target lesion, the fiducial is manually loaded and pushed with the help of a stylet or sterile water. In the second technique, the needle is pre-loaded with one or more fiducials and sealed with sterile bone wax. Subsequently, the fiducials can then be pushed using sterile water or saline. The main complication appears to be pancreatitis and spontaneous migration of markers necessitating a second EUS procedure. In addition, surgically-altered anatomy, such as a previous pancreaticoduodenectomy, may hinder successful placement of fiducial markers. There is no dedicated delivery system for the placement of fiducials under EUS-guidance. Recently, Draganov and colleagues evaluated a novel multi-fiducial delivery system in a porcine model [66]. A prototype 22 G needle pre-loaded with four fiducials was used in this study. Technical success in fiducial deployment was achieved in 96% of study animals and the time for placement of the four fiducials was <1 min (0.86 +/– 0.50 min) [66].
ENDOSCOPIC PALLIATION OF GASTRIC OUTLET OBSTRUCTION
A substantial proportion of pancreatobiliary malignancies have concomitant gastric outlet obstruction due to tumor infiltration. Endoscopic placement of an enteral metal stent is usually effective in these cases and associated with less morbidity as compared to surgical bypass [67-69]. Recently, EUS-guided gastro-enterostomy (EUS-GE) has been reported in patients with malignant gastric-outlet obstruction [70-72]. EUS-GE has been compared with laparoscopic GE and enteral stenting. In a recent multicenter study, EUS-GE was equally efficacious with fewer adverse events as compared to laparoscopic GE [73]. Similar conclusions were drawn in another study, where both the modalities were equal with respect to clinical success, adverse events, and recurrence of obstruction [74]. EUS-GE and enteral stenting were found to have comparable safety and efficacy in one study, but EUS-GE was associated with fewer symptom recurrences and requirements for re-intervention [75]. The stent is deployed away from the site of tumor infiltration with EUS-GE in contrast to enteral stenting. This may theoretically reduce the chances of stent dysfunction due to tumor ingrowth or overgrowth.
EUS-GE can be performed by three different techniques including: (a) direct EUS-GE, (b) assisted EUS-GE using retrieval/dilating balloons, single balloon overtube, nasobiliary drain, and ultraslim endoscope, and (c) EUS-guided double-balloon-occluded gastrojejunostomy bypass [76]. In a recent multicenter study, direct and balloon assisted EUS-GE were compared in patients with gastric-outlet obstruction (2/3rd malignant). Technical and clinical success were similar in both the groups (>90%). However, the procedure duration was shorter with the direct technique (35.7±32.1 minutes vs. 89.9±33.3 minutes, p<0.001) [77].
CONCLUSIONS
Endoscopic palliation is preferred over surgery in a majority of the patients with unresectable pancreatobiliary malignancies. Recent advances in this field include endoscopic ablation in MBO, EUS-guided ablation of locally advanced pancreatic cancers, and the use of EUS for biliary and gastric bypass. These advances are in different phases of development and many of them are not yet ready for general implementation. With the development of dedicated devices and accessories, EUS is likely to play a bigger role in a wide range of palliative procedures in these patients.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.