INTRODUCTION
A gangliocytic paraganglioma is a rare nonepithelial tumor that is histopathologically characterized by a triphasic pattern consisting of epithelioid, ganglion, and spindle-shaped Schwann cells [1]. It usually shows benign features and is located in the second portion of the duodenum. Reportedly, symptoms include abdominal pain, gastrointestinal bleeding [2], and intestinal obstruction (observed in 1 case) [3]. We report a case of a patient in whom esophagogastroduodenoscopy performed during health screening showed an adenoma of the ampulla of Vater, which was treated with endoscopic papillectomy. A gangliocytic paraganglioma was also identified in the duodenal minor papilla (a rare location for this tumor), which was concurrently treated with endoscopic mucosal resection.
CASE REPORT
A 74-year-old woman underwent esophagogastroduodenoscopy during health screening, and discoloration of the ampulla of Vater and a polypoid mass located directly above the ampulla were identified. Biopsy revealed a tubular adenoma with low-grade dysplasia involving the ampulla of Vater, and the polypoid mass showed chronic inflammation. The patient was admitted for treatment.
She had a personal history of AlzheimerŌĆÖs disease and a history of stomach cancer in her brother. Vital signs on admission were unremarkable. She denied gastrointestinal symptoms, and an abdominal examination was unremarkable. Results of laboratory tests including a complete blood count, serum electrolytes, total protein, albumin, total bilirubin, and liver and renal function tests were unremarkable. The carbohydrate antigen 19-9 level was normal.
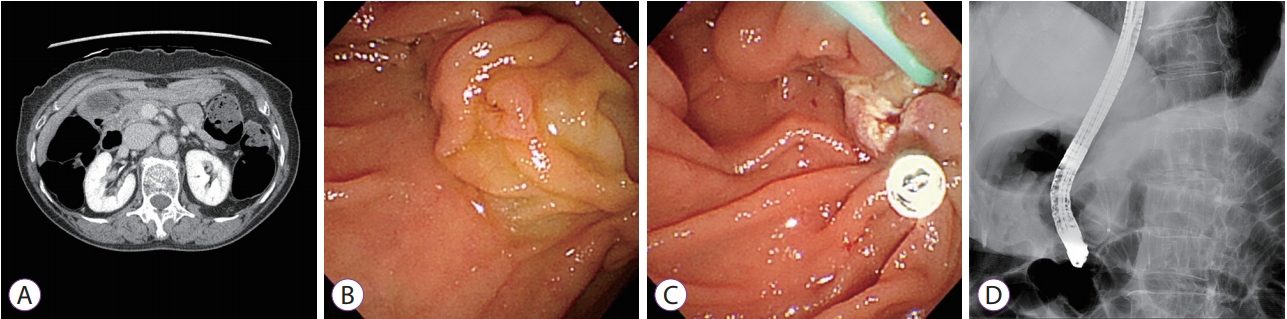
Abdominal computed tomography (CT) performed at another hospital showed no mass at the ampulla of Vater and no definitive bile or pancreatic duct dilatation. Retroperitoneal lymph nodes were not significantly enlarged, and no apparent distant organ site metastasis was identified (Fig. 1A).
The patient provided informed consent before initiation of treatment. Endoscopic examination performed with a side-viewing instrument (TJF-260V; Olympus, Tokyo, Japan) showed a discolored and partially reddish protruding tumor at the ampulla of Vater. After submucosal injection of normal saline, a standard polypectomy snare (Hexagonal, 27-mm; Boston Scientific, Natick, MA, USA) was applied under constant tension, and electrosurgery was performed. En bloc resection was performed, and no residual tumor was observed. After resection, we cannulated the main pancreatic duct and inserted a prophylactic plastic stent (5-Fr ├Ś 3 cm, single pigtail; Cook Medical, Bloomington, IN, USA) to prevent procedure-related pancreatitis. No pancreaticobiliary abnormalities including pancreas divisum were observed during endoscopic retrograde cholangiopancreatography. We used a hemoclip (Hilzo Clip; BCM, Goyang, Korea) to close the papillectomy site to prevent delayed bleeding (Fig. 1B-D).
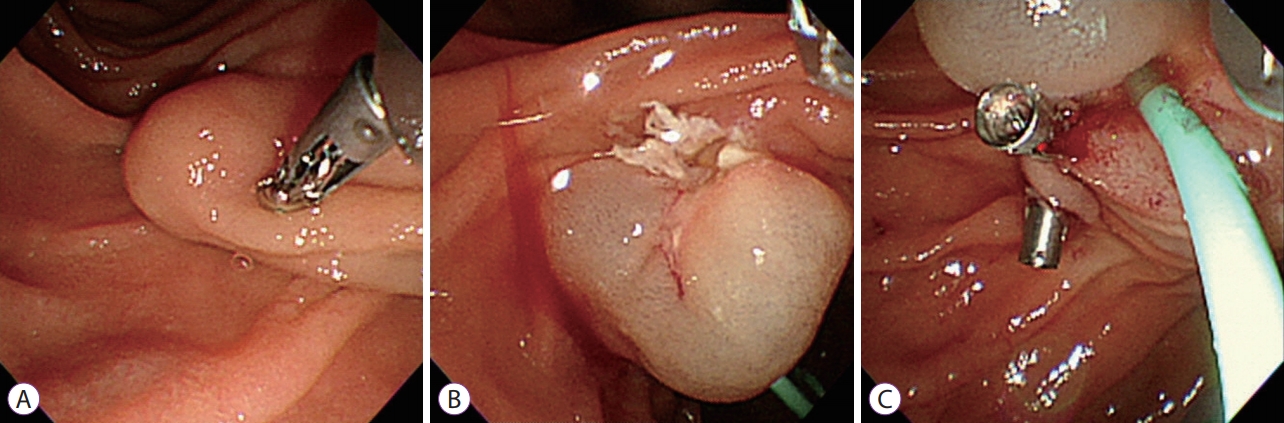
Subsequent endoscopic evaluation of the protruding mass measuring 2 cm in diameter and located directly above the ampulla of Vater showed a normal mucosal surface, indicating that this lesion was a subepithelial tumor. This yellowish tumor was located in the minor papilla.
Pushing on the subepithelial tumor with a biopsy forceps did not produce a rolling sign, and a hard mass with a stalk was identified. On the basis of the size and hardness of the lesion and the absence of lymphadenopathy or distant metastasis on abdominal CT, we decided to remove the tumor using endoscopic mucosal resection. After submucosal injection of saline, we used a standard polypectomy snare and performed complete endoscopic mucosal resection of the subepithelial tumor. Oozing bleeding was observed at the resection site, and we applied a hemoclip to control bleeding (Fig. 2A-C).
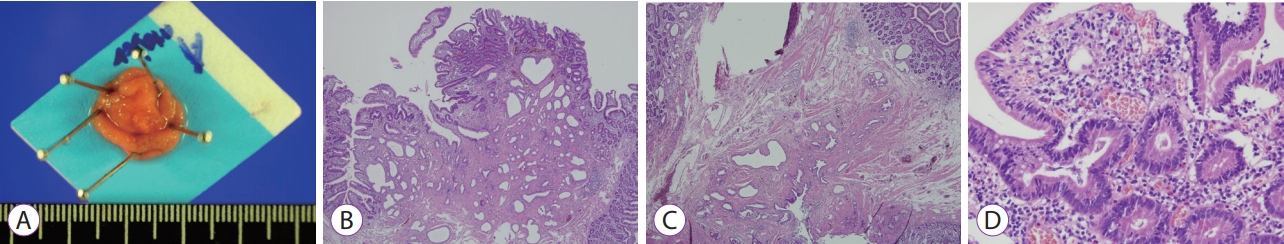
After endoscopic resection, the adenoma at the ampulla of Vater measured 1.7├Ś1.7├Ś0.4 cm in size. Cross-sectional examination revealed a well-demarcated, elevated firm lesion that was confined to the mucosa. Histopathological examination showed dysplastic glandular cells with enlarged, elongated and hyperchromatic nuclei of uniform size, and no loss of polarity, comparable to low-grade dysplasia (Fig. 3).
The subepithelial tumor involving the minor papilla was a well-demarcated, lobulated submucosal lesion that measured 1.8├Ś1.3├Ś0.5 cm in size. The lateral and deep margins were clear. Histopathological examination showed a heterogeneous tumor with 3 components, including Schwann, ganglion, and neuroendocrine cells. The spindle-shaped Schwann cells showed wavy and tapered nuclei, the ganglion cells showed large round nuclei with small nucleoli and finely dispersed chromatin, and the neuroendocrine cells showed a trabeculated pattern (Fig. 4). Immunohistochemical examination showed that the Schwann and ganglion cells stained positive for S-100 protein, the neuroendocrine and ganglion cells stained positive for synaptophysin, and the neuroendocrine cells were also focally and weakly positive for chromogranin stain. Ki-67 staining showed a low proliferation index at <1% (Fig. 5). The subepithelial tumor was therefore diagnosed as a gangliocytic paraganglioma.
The patient did not develop pancreatitis or delayed bleeding after endoscopic resection. She was discharged, and no residual tumor was identified on 6-month follow-up endoscopy.
DISCUSSION
A gangliocytic paraganglioma is considered a neuroendocrine tumor characterized by a triphasic histopathological pattern consisting of epithelioid, ganglion, and spindle-shaped Schwann cells. In most cases, it occurs in the second portion of the duodenum; however, it also occurs in the stomach and the proximal jejunum [1]. It is observed in patients aged between 15 and 84 years (mean age, 52.3 years). Symptoms include abdominal pain, gastrointestinal bleeding, and anemia [2]. A few patients are known to have presented with intestinal obstruction [3], and the lesion was detected in 1 patient during screening esophagogastroduodenosopy [4].
The pathological origin of gangliocytic paragangliomas is unclear. A few authors theorize that the lesion originates from ectopic pancreatic tissue [1], from pluripotent stem cells located at the base of intestinal glands [5], or from endodermally derived epithelial cells in the ventral primordium of the pancreas [6].
The epithelioid cells express neuron-specific enolase, synaptophysin, pancreatic polypeptide, and somatostatin. The ganglion cells express synaptophysin, neuron-specific enolase, and somatostatin. The spindle-shaped Schwann cells express neuron-specific enolase and synaptophysin [2,6].
In most cases, a gangliocytic paraganglioma is located in the second portion of the duodenum, and lesions involving the minor papilla are rare. Matsubayashi et al. analyzed 45 cases of gangliocytic paragangliomas, and only 2 were observed in the minor papilla [7]. In Korea, only a single case report has described a gangliocytic paraganglioma involving the minor papilla [8].
Previously reported cases of a gangliocytic paraganglioma involving the minor papilla are summarized in Table 1 [7-10].
Usually, a gangliocytic paraganglioma shows benign features and progression; however, tumor recurrence or lymph node involvement and distant organ metastasis have been reported in a few cases [11]. Some authors have debated the need for pancreaticoduodenectomy owing to the potential for tumor recurrence and lymph node involvement [12]. However, pancreaticoduodenectomy is associated with a risk of complications, including bile leakage, delayed gastric emptying, and perioperative mortality of 4.1% [13]. Therefore, surgical treatment of a gangliocytic paraganglioma in the duodenum has some limitations.
Barret et al. [14] suggested the following therapeutic approaches for gangliocytic paragangliomas: (1) Tumors measuring <2 cm in diameter without evidence of peritumoral lymph node involvement on abdominal CT can be treated with endoscopic mucosal resection, surgical resection with ampullectomy, or laparoscopic transduodenal tumorectomy combined with preoperative duodenoscopy. (2) Large tumors with suspected lymph node metastasis or histopathological features such as infiltrative margins on local resection, nuclear pleomorphism, or high mitotic activity should be treated with pancreaticoduodenectomy with lymph node dissection [14].
Okubo et al. compared the maximum tumor diameter based on the presence or absence of lymph node metastasis [2]. The group with lymph node metastasis showed a tumor diameter of 5.5ŌĆō65 mm (mean diameter 29.8 mm), and the group without lymph node metastasis showed a tumor diameter of 10ŌĆō100 mm (mean diameter 23.5 mm). No significant difference was observed between the groups [2]. The authors concluded that a treatment plan including surgical or endoscopic resection based on tumor size has its limitations. In contrast, Park et al. suggest that gangliocytic paragangliomas measuring >2 cm can be endoscopically resected, particularly the pedunculated tumor variety [4].
Duodenal tumors include lipomas, leiomyomas, carcinoid, and gastrointestinal tumors with subepithelial characteristics [15]. A gangliocytic paraganglioma also presents with characteristics of a subepithelial tumor; therefore, differential diagnosis is important. Lipomas are yellow with a pillow sign on endoscopy, and endoscopic ultrasound (EUS) examination shows a hyperechoic, homogeneous well-circumscribed mass in the 3rd layer [16]. EUS examination of leiomyomas shows a hypoechoic, well-circumscribed homogeneous lesion in the 2nd or the 4th layer [16]. Carcinoid is typically a firm and yellow sessile nodule observed on endoscopy [15], and EUS shows a hypoechoic mass in the 2nd or the 3rd layer [16]. EUS examination of gastrointestinal tumors usually shows a hypoechoic mass in the 4th layer, but rarely in the 2nd or 3rd layer. Malignant features of gastrointestinal tumors on EUS examination include a cystic component, surface irregularity, and size >3 cm [16,17].
Abdominal CT, EUS, and biopsy can be performed for the diagnosis of gangliocytic paragangliomas. A few reports of EUS findings of gangliocytic paragangliomas have described a well-circumscribed, inhomogeneous hypoechoic mass [4]. A hypoechoic solid mass connected to the submucosal layer has also been reported by a few studies [18], whereas other studies have described that these tumors present as a solid isoechogenic mass [19]. Abdominal CT is useful for the evaluation of lymph nodes and distant metastasis [9]. However, it is difficult to access deep tissues using endoscopic biopsy, and previous studies have reported that only 11.4% of cases were diagnosed on biopsy before treatment [2].
In conclusion, we report a case of a gangliocytic paraganglioma involving the minor papilla (a rare location for this tumor), and the simultaneous occurrence of an adenoma at the ampulla of Vater. No previous case reports have described these tumors. The patient underwent concurrent endoscopic papillectomy and endoscopic mucosal resection.
It is important to consider a gangliocytic paraganglioma in patients presenting with a large subepithelial (<2 cm) duodenal tumor owing to potential lymph node and distant organ metastasis. Endoscopic resection can be performed to treat subepithelial tumors, including gangliocytic paragangliomas if abdominal CT or EUS does not show lymph node involvement or metastasis.