See commentary "Aberrant Expression of Epithelial-Mesenchymal Transition Markers in Early Gastric Cancer: Clinical Application" in Volume 52 on page 393 AbstractBackground/AimsEpithelial-mesenchymal transition (EMT) is a developmental process, wherein the epithelial cells show reduced intercellular adhesions and acquire migratory fibroblastic properties. EMT is associated with downregulation in epithelial marker expression, abnormal translocation of E-cadherin, and upregulation in mesenchymal marker expression. Here, we investigated the immunohistochemical (IHC) expression of EMT markers in early gastric cancer (EGC) between cancer and noncancer tissues.
MethodsTissue samples were prospectively obtained from 19 patients with EGC that underwent endoscopic submucosal dissection (ESD). We compared the expression level of transforming growth factor (TGF)-ЮВ, vascular endothelial growth factor (VEGF), E-cadherin, ЮБ-smooth muscle actin (ЮБ-SMA), and vimentin between cancer and noncancer tissues using IHC. Among the 19 patients, 15 patients had follow-up biopsy at 3 months after ESD for EGC.
INTRODUCTIONEarly gastric cancer (EGC) is defined as a neoplasm confined to the mucosa or submucosa regardless of the presence of regional lymph node metastasis. Endoscopic resection has been widely accepted as the standard treatment for EGC without the risk of regional lymph node metastasis, as it offers advantages of being less invasive and is as effective as surgery [1].
Epithelial-mesenchymal transition (EMT) is a phenomenon that occurs in the normal embryonic development process. In the embryonic stage, EMT is involved in the development of organs as well as in embryo formation. In adults, EMT is associated with wound healing at the time of injury and contributes to general physiological processes such as tissue regeneration. However, EMT is abnormally involved in tissue fibrosis, cancer invasion, drug resistance, and distant metastasis [2,3]. Although EMT plays different roles in tissues, its signal transduction and regulatory mechanisms are similar and particularly related to chronic inflammation and development of cancer. EMT is also shown to be involved in the conversion of chronic inflammation to cancer [4], and is the first process known to be related with the metastasis and progression of cancer. EMT, a process through which the epithelial cells are rearranged during dedifferentiation, plays a key role in the transition of the invasive form of cancers. Several EMT-inducing transcription factors, including, ЮБ-smooth muscle actin (ЮБ-SMA), transforming growth factor (TGF)-ЮВ, and vascular endothelial growth factor (VEGF) are known to induce spreading and morphological changes in cancer cells through the suppression of E-cadherin and induction of mesenchymal markers [5,6]. However, most previous studies have been conducted in patients with advanced gastric cancer (AGC) that underwent surgery; hence, the implication of EMT in patients with EGC remains unclear.
During EMT, epithelial cells undergo a phenotypic switch to form mesenchymal cells that are similar in appearance to fibroblasts [7,8]. EMT, characterized with a gain of mesenchymal cell markers and loss of epithelial markers,is a process that activates cell motility and invasion [9,10]. EMT plays an important role in the formation, invasion, and metastasis of gastric cancer, and EMT-related proteins have been suggested to interact with each other in various cancers and are associated with the aggressive behavior of cancers.
Studies have been conducted on patients that underwent surgery for AGC or who have had metastasis [11,12]; however, limited information is available on the immunohistochemical (IHC) expression of EMT markers in cancer and noncancer tissues of patients with EGC. The molecular profiles of metastatic cancer were thought to be similar to those of primary cancer because metastases are derived from their primary counterparts. It is well known that EMT mainly appears in AGC, and these markers are helpful for predicting patient prognosis. To demonstrate the clinical significance of EMT-related IHC markers in EGC and differences in their expression patterns between cancer and noncancer tissues, we performed IHC staining using samples obtained from 19 patients with EGC.
MATERIALS AND METHODSPatientsA prospective study was conducted on patients; the details of the study were explained to each patient and informed consent to participation was obtained before the study inception. Patients underwent endoscopic submucosal dissection (ESD) after being diagnosed with EGC at Soonchunhyang University Hospital in Bucheon within the period from May 2014 to September 2015. After enrollment in this study, the clinical records and tissue slides of the patients were reviewed, and the age, sex, tumor size, histologic subtype, and lymphatic metastasis were analyzed. The implementation direction of this study is summarized in Fig. 1. Before commencement, the study was approved by the Institutional Review Boards of Soonchunhyang Medical Center (IRB No. 2016-12-015-003). Written informed consent was obtained from patients before the study-related interview was performed. The obtainment of consent was confirmed by IRB.
Definition of cancer tissue and noncancer tissueThe tissue that was collected around the margin of the cancer during endoscopic treatment was defined as тcancer tissueт. The tissue that was located at a distance of more than 5 cm from the margin of the tumor was defined as тnoncancer tissueт [13]. The tissue collected around the ESD scar 3 months after the endoscopic procedure was defined as тNONCANCER TISSUEт. According to these definitions, we compared the differences in EMT markers between тcancer tissue versus noncancer tissueт and тcancer tissue versus NONCANCER TISSUEт.
Optical microscopic examinationPrior to inclusion in the study, all cases were reviewed by two experienced medical doctors (one pathologist, and one gastroenterologist) for confirmation of diagnosis, staging, and grading. This was a blinded study and the patientsт clinical characteristics were unknown to the investigators performing IHC analyses. In case of discrepancy in the interpretation of the same slide, the evaluation was repeated until a consensus was obtained. The tissues to be observed were fixed with 10% neutral formalin for 24 h and rinsed with distilled water for 20 min. After dehydration with ethyl alcohol, the tissues were washed with xylene. Paraffin blocks prepared through paraffin-embedding process were cut into 4-mm-thick samples (Muto, Tokyo, Japan) and subjected to deparaffinization. The samples were soaked in ethyl alcohol and washed, and subjected to hematoxylin and eosin (H&E) staining. According to the histologic findings of H&E staining, representative sites were examined with an optical microscope, and slides were prepared for the IHC staining of these sites. The histologic classification of tumors was based on the Lauren classification system as well as depending on the presence of vascular or lymphatic metastasis.
IHC stainingThe samples cut from the paraffin-embedded block were immersed in xylene solution for 30 min to remove the paraffin, and subjected to successive treatment with 100%, 90%, and 70% ethyl alcohol. After being washed thrice with phosphate-buffered saline, the samples were treated with 3% hydrogen peroxide for 10 min to remove any endogenous peroxidase. The samples were boiled in a citric buffer solution (pH 6.0) for 40 min and cooled for 25 min to expose the antigen. To inhibit non-specific binding of the proteins, antibodies against TGF-ЮВ (diluted 1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), VEGF (diluted 1:200, Santa Cruz Biotechnology Inc.), E-cadherin (diluted 1:150, Santa Cruz Biotechnology Inc.), ЮБ-SMA (diluted 1:100, Abcam Plc, Cambridge, UK), and vimentin (diluted 1:300, Sigma Aldrich Inc., Saint Louis, MO, USA) were used as primary antibodies after they were reacted with a blocking antibody for 10 min. The samples were incubated at 37ТАC for 1 h and washed thrice with Tris-buffered saline (TBS). The samples were treated with a biotin-conjugated secondary antibody (Dako, Glostrup, Denmark) for 10 min, washed with TBS, and treated with streptavidin-biotin conjugate for 10 min. Color development for amino-ethyl carbazole (Zymed, San Francisco, CA, USA) was performed after washing with TBS. The samples were stained with 10% Mayerтs hematoxylin (Biomedia, Foster City, CA, USA) and subjected to encapsulation into inclusion bodies and microscopic observation. For the in-house control group, normal sheep serum was added instead of primary antibody after staining, and the rest of the staining process was repeated, as mentioned above.
IHC staining assessmentThe results of IHC staining were judged by comparison with the in-house control group, including the normal epithelial tissue. TGF-ЮВ, VEGF, ЮБ-SMA, and vimentin expression was deemed positive when the nuclei of the tumor cells were stained reddish brown. E-cadherin expression was considered positive when the cells were stained reddish brown along the cytoplasm or cell membrane. For each case, the number of tumor cells was counted at У200 magnification under an optical microscope, and the number of positively stained cells was counted. The expression of TGF-ЮВ, VEGF, ЮБ-SMA, and vimentin was deemed positive if more than 5% of the cells showed positive staining. E-cadherin expression was deemed positive when the proportion of stained cancer cells was 25% or more among all cancer cells [11,12]. EMT was considered to be positive if the expression of E-cadherin disappeared or that of other markers was present.
Assessment of Helicobacter pylori infection
Helicobacter pylori infection was diagnosed by obtaining the tissues with biopsy forceps via upper gastrointestinal endoscopy. The obtained tissues were subjected to modified Giemsa staining or rapid urease test (Pronto DryТЎ, hereafter, тRUTт). The positivity of either of these tests indicated H. pylori infection. In the RUT, the tissue sections obtained from the endoscopic procedures were stored in an RUT vessel, and the results were evaluated after 1 h at room temperature (24ТАC). The yellow color of the paper used in RUT even after 24 h indicated the absence of H. pylori infection. The red color of the paper, on the other hand, confirmed H. pylori infection. For modified Giemsa staining, the collected tissues were fixed with 10% formalin solution, and a paraffin block was made and cut into slices (4 ЮМm thickness). The presence of infection was determined according to the colonization of H. pylori.
Statistical analysisThe expression of TGF-ЮВ, VEGF, E-cadherin, ЮБ-SMA, and vimentin proteins was compared between the cancer and noncancer tissues. For various clinicopathological factors such as tumor location, H. pylori infection, and IHC staining of EMT markers after tumor removal, all the continuous variables were summarized with the meanТБstandard deviation and analyzed through the independent sample t-test. The categorical variables were compared through the chi-square test and Fisherтs exact test. Statistical analysis was performed using SPSS ver. 14 (SPSS Inc., Chicago, IL, USA), and p<0.05 was considered statistically significant.
RESULTSCharacteristics of patientsAmong the patients that had undergone ESD for EGC, 19 (9 males, 10 females) were included in this study. The mean age of the patients was 60 years, and the mean size of the tumors was 24.6 mm. Characteristics of the patients are summarized in Table 1.
Differences in EMT between the cancer and noncancer tissuesAnalysis of E-cadherin expression showed that the cells from the noncancer tissues were stained along the cell membrane and had a trend of decreased E-cadherin expression when differentiation was poor. The expression of TGF-ЮВ, VEGF, ЮБ-SMA, and vimentin increased in the cancer tissues as compared to that in the noncancer tissues (Fig. 2). No statistically significant difference was observed with respect to E-cadherin expression between the two groups. Two cases of poor differentiation and three cases of moderate differentiation were observed among cancer tissues for E-cadherin expression and all of these samples were stained at well-differentiated adenocarcinoma. In terms of the association with tumor differentiation, E-cadherin loss was related to poor differentiation grade; it was difficult to make any statistical judgment, owing to the small sample size. TGF-ЮВ, VEGF, ЮБ-SMA, and vimentin were overexpressed in the cancer tissues as compared to the noncancer tissues and statistically significant difference was reported (Table 2).
IHC for EMT markers near the ESD scar after tumor removal (NONCANCER TISSUE)IHC for EMT markers was performed with the ESD scar tissues (called тNONCANCER TISSUEт) and compared with the initial cancer tissue. IHC expression of EMT markers after the removal of tumor was similar to that observed in the initial noncancer tissue. The results of cancer tissue versus NONCANCER TISSUE were similar to those of cancer tissue versus noncancer tissue (Table 3).
Differences in EMT marker expression according to the location of the tumorTo investigate whether there exists any difference in the expression of EMT markers based on the location of the tumor, the tumors were divided as follows: a tumor both in the proximal 1/3 and middle 1/3 region and a tumor in the distal 1/3 region. In all the cases, no significant difference was observed in the overexpression of TGF-ЮВ (p=0.615), VEGF (p=0.912), ЮБ-SMA (p=0.503), and vimentin (p=0.503) and loss of E-cadherin (p=0.720) based on location.
Differences in EMT marker expression according to H. pylori infectionTo investigate the differences in the expression of EMT markers based on H. pylori infection, we divided the samples into those with and without H. pylori infection. No significant difference in EMT was observed in TGF-ЮВ (p=0.754), VEGF (p=0.737), ЮБ-SMA (p=0.754), and vimentin (p=0.754) overexpression and E-cadherin loss (p=0.918) according to H. pylori infection.
DISCUSSIONIn the present study, we investigated the IHC expression of EMT markers in EGC. We aimed to analyze (1) IHC expression of EMT markers in patients that underwent ESD with EGC (previous study, expression of EMT markers in patients that underwent surgery with AGC) and (2) any difference in EMT markers between noncancer and cancer tissues based on the pathologic examination without MicroRNA (miRNA) test. We compared the pathologic differences in EMT between the samples collected from the near margin of the cancer and those obtained from the distant tissues during endoscopic treatment (cancer tissue versus noncancer tissue). We also compared the pathologic differences in EMT between the tissues collected from the near margin of the cancer during endoscopic treatment and those obtained from ESD scar 3 months after ESD (cancer tissue versus NONCANCER TISSUE). The results of this study revealed the pathologic differences in EMT in patients with EGC. IHC analysis revealed the significant difference in mesenchymal markers (such as TGF-ЮВ, VEGF, ЮБ-SMA, vimentin) between cancer and noncancer tissues.
The expression of the epithelial marker E-cadherin was not significantly different between cancer and noncancer tissues. Considering the reason, E-cadherin loss was more prominent in poor differentiated, well-differentiation was more common in ESD patients [14]. We did not compare the difference in E-cadherin loss according to the degree of differentiation in patients with EGC, owing to the small sample size of this study. Many reports have been published on the expression of E-cadherin and tumor progression. In AGC, E-cadherin expression disappears upon poor histological differentiation and falls under the Lauren classification of diffuse type and is associated with lymphovascular metastasis. In the present study, two patients with poor differentiation showed loss of E-cadherin expression, but it was difficult to perform statistical analysis owing to the small number of cases. According to a recent report, loss of E-cadherin expression and overexpression of VEGF are more common with poor histological differentiation in patients with EGC [15].
EMT is a conserved cellular program that alters cell shape, adhesion, and movement. The shift to a more mesenchymal-like phenotype may promote intravasation of tumor cells in the surrounding blood vessels and their emigration to a new organ. Carcinomas are tumors of epithelial origin. As carcinoma cells are genetically unstable, they do not start from a purely normal epithelial phenotype but rather from a somewhat activated phenotype. miRNAs may influence multiple steps in cancer cell metastasis and are well established as key regulators of the EMT program in epithelial cells. EMT is a complex process that may be influenced by different pathways; thus, EMT may be regulated by many miRNA-mediated mechanisms. miRNAs can directly bind and suppress transcription factors, suppress transcription of key EMT molecules such as vimentin and E-cadherin, or affect epigenetic regulators of EMT. Based on the target molecules that they bind and suppress, miRNAs may promote EMT and therefore suppress mesenchymal-epithelial transition (MET) or promote MET and consequently inhibit DNA. As it is difficult to detect EMT with a mere pathologic examination, it is appropriate to evaluate EMT with miRNA tests. However, the aim of this study was to compare IHC expression of EMT-associated markers without miRNA test, assuming that EMT marker expression differs between cancer tissue and noncancer tissue (distant to cancer tissue & scar site after removal of the cancer tissue). This study was conducted to evaluate the difference in pathologic examination alone because miRNA test is generally difficult to perform in all patients, owing to high cost and other factors. A previous study with cancer and noncancer tissues from patients that underwent surgery for AGC reported an association between RNA methylation and IHC expression [16].
EMT is the first stage of the invasion and metastasis of cancer cells, wherein the epithelial cells that cannot migrate to other parts of the body due to cell-to-cell junctions form collagen fibers and get transformed into mesenchymal cells that can easily migrate to the other parts of the body [4,17]. While EMT occurs within the cells, E-cadherin expression that is essential for the inhibition of epithelial-cell migration and for maintenance of cell shape, disappears. On the contrary, the expression of vimentin, ЮБ-SMA, and VEGF, which are the proteins necessary for cell mobility, increases [18,19]. E-cadherin expressed in the epithelial cells functions to closely adhere the tissue membranes to the cells. The transcription factor TGF-ЮВ inhibits the expression of E-cadherin and induces EMT, which is involved in cancer cell metastasis through the promotion of cellular motility [20]. In terms of research on EMT in gastric cancer, many studies have been conducted on the surgically resected gastric cancer or AGC tissues with lymph node or vascular metastasis [11,12]; no report on endoscopically resectable EGC is available. Therefore, in the present study, we investigated the difference in the expression of EMT markers between cancer and noncancer tissues in patients with EGC and evaluated the IHC expression of EMT markers around the primary cancer tissue scar after the complete resection of the tumor (NONCANCER TISSUE).
Vimentin/E-cadherin ratio is an important factor in the prediction of the prognosis of gastric cancer in relation to EMT [6]. Prognosis is reported to be worse with the progression of AGC when differentiation is poorer or when the histologic subtype is diffuse type [21]. Prognosis can be carried out with the identification of these factors. The loss of E-cadherin expression or the overexpression of vimentin and ЮБ-SMA is associated with the depth of tumor invasion, lymph node or distant metastasis, grade of histological differentiation, and tumor, node and metastasis classification stage. In cancer tissues, E-cadherin expression disappears or vimentin and ЮБ-SMA are significantly overexpressed [22]. E-cadherin, a marker of epithelial cells, retains its original trait and is expressed in the cell membrane, but the expression disappears when EMT occurs in advanced or poorly differentiated cancers. E-cadherin plays a key role in EMT and is associated with the adherence junctions. EMT also plays a major role in cancer metastasis and is a complex, multifunctional, and tightly regulated developmental program. The understanding of the different strategies employed by the tumor cells to switch EMT on and off is important.
MET is a reverse process that plays an important role in the formation of epithelium. It is now widely known that EMT constitutes to the early metastatic step, wherein the cells that have undergone EMT may detach from the primary tumor, invade through the basement membrane into the circulation, and revert back to the epithelial phenotype to induce metastasis at a distant secondary site. It is well known that miRNAs are important regulators of malignant transformation and metastasis. miRNAs regulate EMT and control major signaling pathways in various cancers. Various miRNAs are reported to directly target EMT transcription factors and components of the cell architecture. In addition, these miRNAs may also reverse the EMT process [23].
The limitations of this study are as follows. First, we evaluated the expression of EMT markers only by IHC. In a previous study, the miRNA expression of EMT markers was evaluated, thereby reinforcing the validity of the study results [21]. As tumors are genetically unstable, some pathologists suggest the unreliability of IHC markers while a few reported the absence of EMT markers in pathology [24]. Nevertheless, in this study, pathologic differences in the expression of EMT markers were significant between noncancer and cancer tissues. Second, the limited number of patients included in the study posed difficulties in establishing statistical significance or to generalize the result because sufficient consent and co-operation had to be obtained from the patients. Further research is warranted while addressing the limitations of the present study to help in the early detection of gastric cancer and for the assessment of cancer prognosis.
In conclusion, mesenchymal markers of EMT, such as TGF-ЮВ, VEGF, ЮБ-SMA, and vimentin, may serve as potential targets for EGC; however, further evidence is needed to confirm this finding.
Fig.Т 1.Flow chart of study design. ЮБ-SMA, ЮБ-smooth muscle actin; EGC, early gastric cancer; ESD, endoscopic submucosal dissection; F/U, follow-up; IHC, immunohistochemical; TGF-ЮВ, transforming growth factor-ЮВ; VEGF, vascular endothelial growth factor. 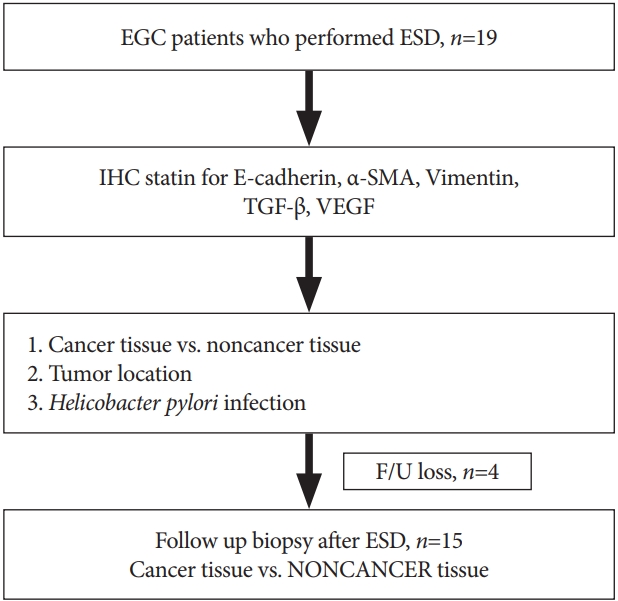
Fig.Т 2.Immunohistochemical staining of noncancer tissue and cancer tissue. Representative expression of proteins studied by immunohistochemistry (original magnification У200). This figure shows noncancer tissue and cancer tissues. (A, B) transforming growth factor-ЮВ, (C, D) VEGF, (E, F) E-cadherin, (G, H) ЮБ-smooth muscle actin, and (I, J) vimentin. 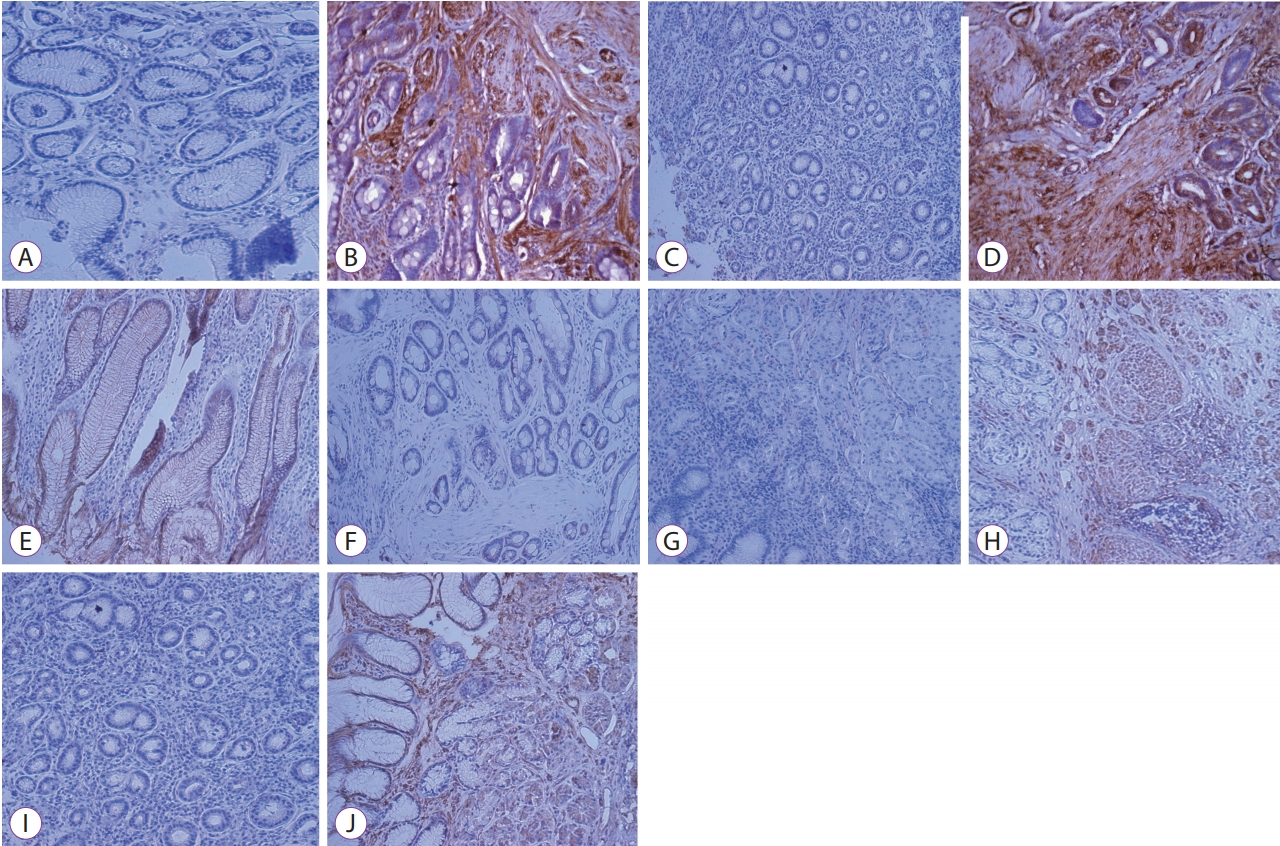
TableТ 1.The Baseline Characteristic of Patients TableТ 2.Differences of Epithelial-Mesenchymal Transition between Cancer Tissue and Noncancer Tissue TableТ 3.Differences of Epithelial-Mesenchymal Transition between Cancer Tissue and NONCANCER TISSUE REFERENCES1. Lee HJ, Lee YJ, Lee JY, et al. Characteristics of synchronous and metachronous multiple gastric tumors after endoscopic submucosal dissection of early gastric neoplasm. Clin Endosc 2018;51:266т273.
2. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871т890.
3. Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol 2008;14:3792т3797.
4. LУГpez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009;1:303т314.
5. Zhu Y, Wu J, Ma W, Zhang H, Wang D. Expression of TGF-ЮВ1, Snail, E-cadherin and N-cadherin in gastric cancer and its significance. Chinese Journal of Clinical Oncology 2007;4:384т389.
6. Murai T, Yamada S, Fuchs BC, et al. Epithelial-to-mesenchymal transition predicts prognosis in clinical gastric cancer. J Surg Oncol 2014;109:684т689.
7. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006;7:131т142.
8. Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev 2003;120:1351т1383.
10. Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res 2006;66:4549т4552.
11. Ryu HS, Park DJ, Kim HH, Kim WH, Lee HS. Combination of epithelial-mesenchymal transition and cancer stem cell-like phenotypes has independent prognostic value in gastric cancer. Hum Pathol 2012;43:520т528.
12. Xu GF, Zhang WJ, Sun Q, Xu X, Zou X, Guan W. Combined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancer. World J Surg Oncol 2014;12:368.
13. Xiao Q, Li L, Xie Y, et al. Transcription factor E2F-1 is upregulated in human gastric cancer tissues and its overexpression suppresses gastric tumor cell proliferation. Cell Oncol 2007;29:335т349.
14. LazФr D, TФban S, Ardeleanu C, et al. The immunohistochemical expression of E-cadherin in gastric cancer; correlations with clinicopathological factors and patientsт survival. Rom J Morphol Embryol 2008;49:459т467.
15. Han JP, Hong SJ, Kim HK, et al. Expression of immunohistochemical markers according to histological type in patients with early gastric cancer. Scand J Gastroenterol 2016;51:60т66.
16. Cong H, Yao RY, Sun ZQ, et al. DNA hypermethylation of the vimentin gene inversely correlates with vimentin expression in intestinal- and diffuse-type gastric cancer. Oncol Lett 2016;11:842т848.
17. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442т454.
18. Nakayama H, Enzan H, Miyazaki E, Toi M. Alpha smooth muscle actin positive stromal cells in gastric carcinoma. J Clin Pathol 2002;55:741т744.
19. Grigore D, Simionescu CE, Stepan A, et al. Assessment of CD105, alpha-SMA and VEGF expression in gastric carcinomas. Rom J Morphol Embryol 2013;54(3 Suppl):701т707.
20. Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res 2015;7:2141т2158.
21. Choi YJ, Kim N, Chang H, et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015;36:553т563.
|
|
||||||||||||||||||||||||||||||||||||||||||||
