INTRODUCTION
While endoscopic submucosal dissection (ESD) is an established treatment method for gastric adenoma and early gastric cancer [1], it remains a suboptimal option for the treatment of a subgroup of lesions such as subepithelial tumors arising from the muscularis propria. Performing ESD for such tumors often results in low rates of microscopically margin-negative (R0) resection and has a higher risk of perforation [2-4]. When lesions are situated at difficult anatomical locations to access, ESD can result in high rates of adverse events. In view of these challenges, endoscopic full thickness resection (EFTR) is the preferred resection technique for this subgroup of tumors.
In this review, we discuss the indications for EFTR. We also describe the technical details of various EFTR techniques, including their advantages and disadvantages. Finally, we discuss the current challenges among endoscopists who are keen to adopt EFTR in their clinical practice, and how our novel robotic endoscopic platform with suturing capabilities can overcome these technical challenges.
INDICATIONS FOR ENDOSCOPIC FULL THICKNESS RESECTION
EFTR is especially useful in the management of lesions where ESD produces suboptimal results.
Lesions arising from or infiltrating the deeper layers of submucosa or muscularis propria
EFTR is especially beneficial in the treatment of submucosal tumors. These include gastrointestinal subepithelial tumors and T1 carcinoma with no lymphatic and/or vascular invasion. Tumors that infiltrate into the muscularis propria, such as gastrointestinal stromal tumors (GISTs), leiomyoma and schwannoma, can also benefit from EFTR.
A retrospective study conducted by Kuellmer et al. in 156 patients, showed that EFTR is technically feasible and safe in cases of early colorectal cancer identified via histology [5]. Technical success and R0 resection were achieved in 92.3% and 71.8% of the cases respectively. Severe procedure-related adverse events occurred in only 3.9% of patients [5].
Non-lifting lesions
Non-lifting lesions can occur due to fibrosis or scarring. They can also develop due to recurrence of epithelial neoplasms following endoscopic mucosal resection or ESD procedure [6]. In earlier times, non-lifting lesions were a contraindication for standard polypectomy. However, with the introduction of EFTR, non-lifting lesions can now be resected with high rates of R0 resection and low rates of perforation.
A retrospective study conducted by Kuellmer et al. showed that the rate of R0 resection was 60.9% in the group with non-lifting lesions [5]. The final histology revealed adenocarcinoma in 100% of the specimens.
Lesions at difficult anatomical locations to access
Resection of the lesions situated at the appendiceal orifice or within the diverticulum can be extremely challenging using conventional techniques. Using EFTR to treat these lesions can lower the rates of adverse events, particularly iatrogenic perforations.
In the upper gastrointestinal tract, ESD is more challenging for lesions located in the cardia or along the lesser curvature, due to the need for retroflexion. Lesions in the gastroduodenal region also pose significant challenges to the endoscopist. For these lesions, the rate of adverse events can be lowered by using EFTR.
TECHNIQUES FOR ENDOSCOPIC FULL THICKNESS RESECTION
Since the introduction of EFTR, numerous techniques have been developed. We describe here, the brief technical details of each technique, and provide evidences of the advantages and disadvantages of each method. Table 1 can be used as a roadmap for endoscopists to decide which technique is best suited for their patient, depending on the tumor size and the availability of equipment and expertise.
A. Non-tunneling techniques
Endoscopic submucosal excavation
Technical details
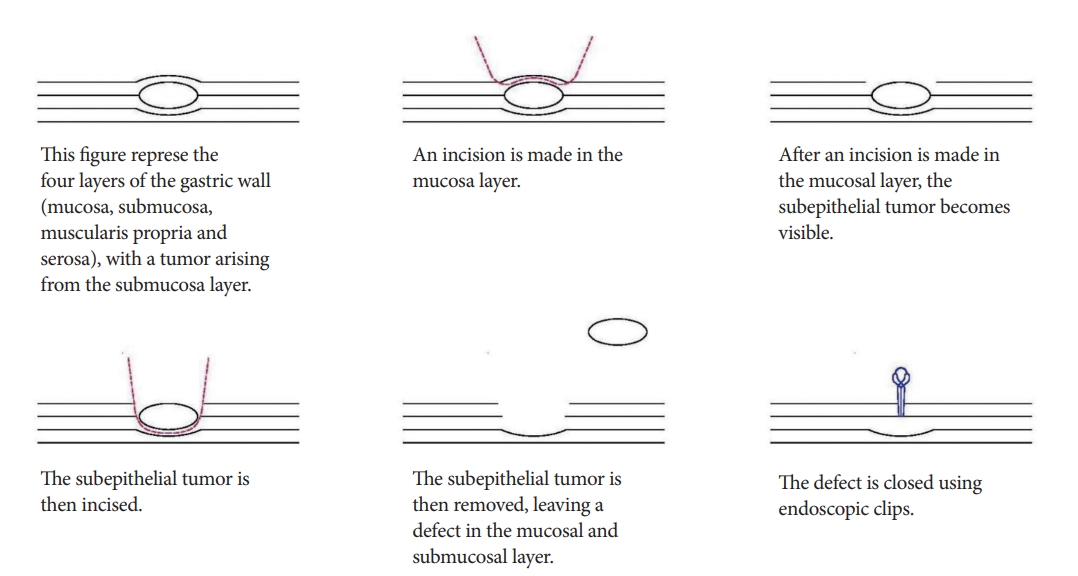
In endoscopic submucosal excavation (ESE), no circumferential incision is performed. Instead, the mucosa overlying the lesion is incised to allow the subepithelial tumor to be dissected from the submucosal or muscularis propria layer. Subsequently, endoscopic clips are used to close the site of incision (Fig. 1) [7].
Advantages
ESE has been used effectively in the treatment of esophageal and gastric subepithelial tumors. R0 resection rates with ESE are higher compared to the traditional ESD techniques. Ye et al. showed that 95.6% of small esophageal subepithelial tumors originating from the muscularis propria could be successfully resected with negative margins using ESE [8]. The high rate of success of ESE for resections of gastric subepithelial tumors was also demonstrated by Jeong et al. [9].
Disadvantages
Despite the high R0 resection rates, perforation rates remain as high as 13% [10]. During resection, there is loss of air insufflation, resulting in collapse of the wall of the gastrointestinal tract, and a significantly restricted view of the operative field. The analysis by Jeong et al. showed that perforations were more commonly associated with GISTs and schwannomas, as these tumors had incomplete tumor capsules and were tightly adherent to the surrounding tissue [9]. In addition, the relatively thin wall of the gastric fundus and its location are a challenge for endoscopic access. This predisposes fundal tumors to higher rates of perforation, compared to other sites in the stomach (p<0.001) [9].
Endoscopic full-thickness resection with secondary closure (exposed EFTR)
Technical details
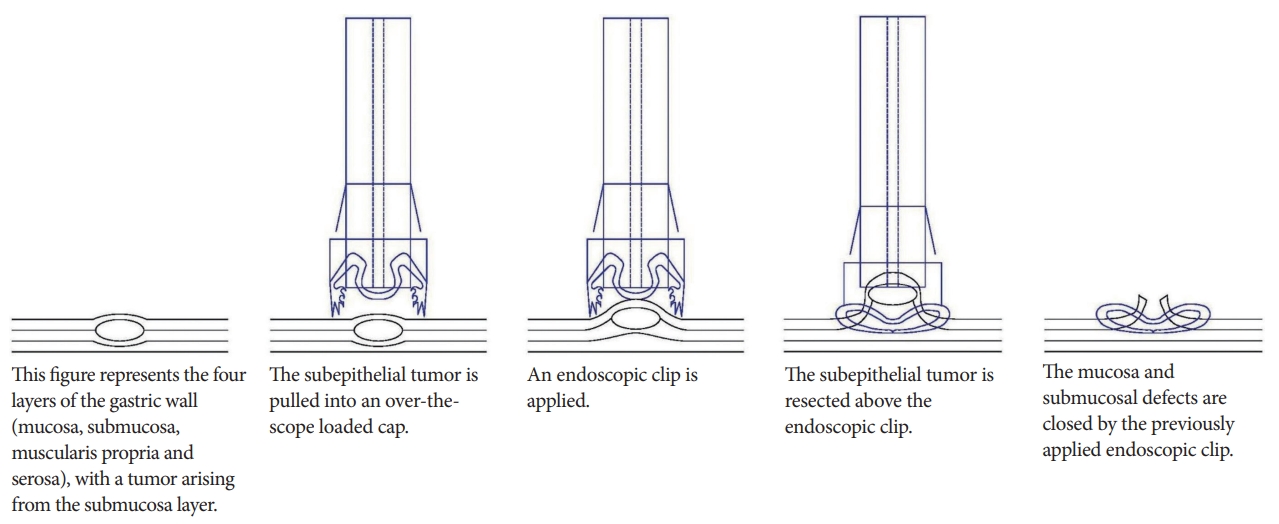
To reduce the rates of perforation, Kuellmer et al. developed a technique for performing exposed EFTR with secondary closure [5]. When performing exposed EFTR, a full thickness resection device such as the Oversco Endoscopy is required. The lesion is first marked, and is then grasped and pulled into the cap, attached above the scope. The lesion is resected after applying the clip (Fig. 2).
Disadvantages
As seen in all exposed EFTR techniques, there is a risk of intraperitoneal contamination by the gastric contents, and dissemination of tumor cells within the peritoneal cavity [11]. Moreover, if the size of the tumor is large, closure of the defect is technically challenging. Current endoscopic instruments are not ideally suitable for performing full-thickness suturing while maintaining insufflation of the gastrointestinal tract.
B. Tunneling techniques
Submucosal tunneling with endoscopic resection
Technical details
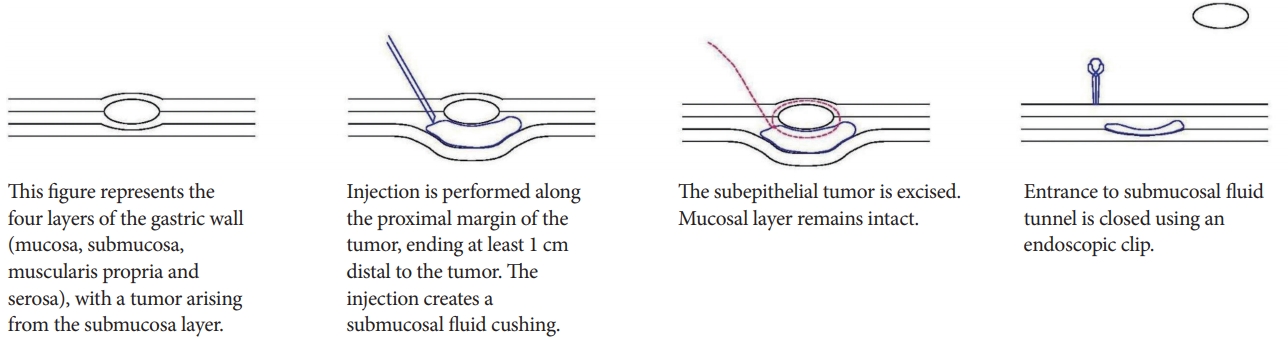
In submucosal tunneling with endoscopic resection (STER), a submucosal fluid cushion is created by injecting a mixed solution of normal saline, indigo carmine, and epinephrine, along the proximal margin of the tumor. This creates a submucosal tunnel between the mucosal and muscular layer. This submucosal tunnel should ideally end 1 cm distal to the tumor, to ensure adequate working space and satisfactory endoscopic view of the tumor. The crux of the STER procedure is the creation of a mucosal incision with submucosal tunneling of the endoscope along the fluid cushion. This ensures that there remains an intact cover of mucosa during the resection. The tumor is then enucleated and extracted. After hemostasis is performed, the entrance to the submucosal tunnel is closed with endoscopic clips (Fig. 3) [12].
Advantages
A retrospective study of 180 patients was performed by Chen et al. [12]. All patients had upper gastrointestinal subepithelial tumors that were resected using the STER technique. Only 1 patient required additional surgery. Adverse events were encountered in 8.3% of the patients, with no severe mediastinal or intra-abdominal infections. Of these, 177 patients were followed up for a median duration of 36 months, and all of them were free from local recurrences or distant metastasis. Thus, their study showed that STER is an effective and safe resection technique for upper gastrointestinal subepithelial tumors.
The use of a submucosal tunnel in STER also reduces the likelihood of intraperitoneal contamination by gastric contents, and minimizes the likelihood of intraperitoneal tumor cell dissemination [6]. This promotes faster wound healing and patient recovery.
Disadvantages
In certain parts of the gastrointestinal tract, creation of a submucosal tunnel might be anatomically challenging [6]. In such cases, it would be more appropriate to perform non-tunneling EFTR methods.
C. Collaborative techniques
One of the most common collaborative techniques between surgeons and endoscopists is laparoscopic and endoscopic cooperative surgery (LECS). LECS is an accepted technique of minimally invasive laparoscopic wedge resection, and is commonly used during surgery for GISTs.
Advantages
The main advantage of LECS is its precise determination of the tumor boundary on endoscopy. This allows for R0 resection while minimizing the amount of gastric tissue resected. This leads to functional and anatomical preservation of the gastrointestinal tract [12].
Technical details
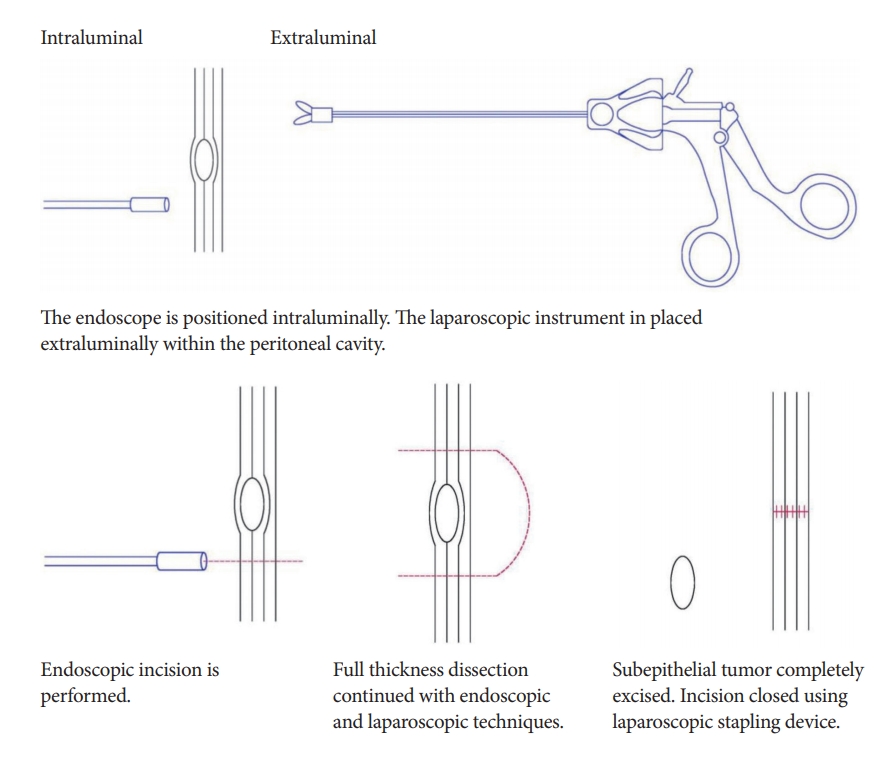
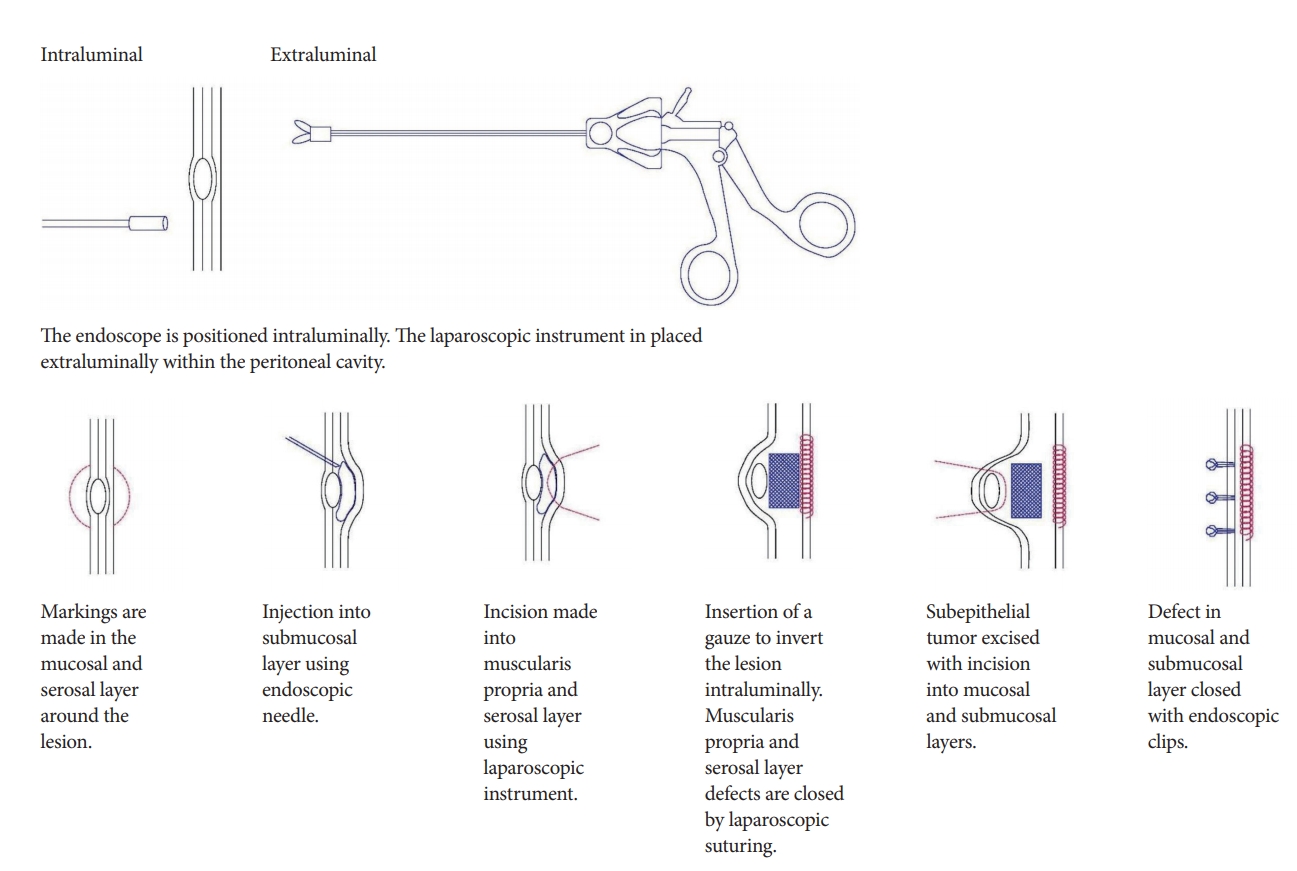
To prevent spillage of the gastric intraluminal contents into the peritoneal cavity, Mitsui et al. described a type of LECS called non-exposed endoscopic wall-inversion surgery (NEWS) [14]. In NEWS, markings are made on the mucosa around the lesion, as well as on the serosal surface. The submucosal layer is then injected with a solution and circumferential seromuscular layer incision is performed. Suturing is then performed in the seromuscular space with inversion of the lesion using a gauze spacer, causing protrusion of the gastric mucosa due to the inverted tissue. The muco-submucosal layer is then incised and endoscopic clips are placed to close the artificial linear ulcer. The specimen is then extracted orally using an endoscopic retrieval device. The crux of NEWS is to first place the sutures to secure the resection site, and then complete the resection (Fig. 5).
Advantages
With suturing performed prior to resection, it reduces the likelihood of peritoneal contamination by intragastric contents, and minimizes the dissemination of tumor cells. This overcomes the innate flaw of LECS with its deliberate gastric perforation.
Mitsui et al. described the use of NEWS in 28 patients with gastric GISTs [14]. NEWS was found to be feasible for the treatment of small GISTs [14]. NEWS is especially suitable for small GISTs with mucosal ulceration, where opening of the gastric wall by full-thickness enucleation would not be optimal.
CHALLENGES IN ENDOSCOPIC FULL THICKNESS RESECTION
Despite the advancement in EFTR techniques, EFTR has not been adopted in routine endoscopic practice. An international survey conducted by Goto et al. in 2016 (personal communication) attempted to elucidate the reasons for the lack of wide adoption of this technique. Sixty percent of the respondents indicated that they had direct experience in performing endoscopic resection under laparoscopic assistance. However, 32% of the respondents indicated by way of personal communication that laparoscopic closure by hand-suturing was technically difficult and/or very difficult.
SOLUTIONS TO OVERCOME THE TECHNICAL CHALLENGES IN ENDOSCOPIC FULL THICKNESS RESECTION
Due to the technical difficulties in performing laparoscopic and endoscopic suturing using the currently available instruments, it is paramount to identify what an ideal EFTR system should encompass. The ideal EFTR system should have two componentsāfirst, the system should allow for good maneuvering and triangulation; second, the system should allow for safe and reliable closure of any gastrointestinal tract defects.
Currently, there are four FDA approved endoscopic suturing devicesāInScope Tissue Apposition System (Ethicon EndoāSurgery, Cincinnati, OH, USA), g-Prox Tissue Approximation Device (USGI; USGI Medical, San Clemente, CA, USA), OverStitch (Apollo Endosurgery, Austin, TX, USA), and OTSC (Ovesco Endoscopy, Tuebingen, Germany). However, the major challenge of these mechanically driven platforms is that they do not allow for hand suturing with serosa-to-serosa apposition.
Recently, Endomina (Endotools SA, Gosselies, Belgium), a single-use triangulation robotic platform was introduced for endoluminal suturing. However, the two operating arms are inserted over-the-scope using guidewires, instead of through the existing endoscopic channels. This results in a large and bulky instrument with potential risk of trauma to the upper gastrointestinal tract during insertion.
We have developed a Master and Slave TransEndoluminal Robot (MASTER) (EndoMaster Pte. Ltd., Singapore), which is equipped with a multitasking robotic endoscopic platform and interchangeable robotic arms (Fig. 6). The major benefit of this system is the usability of the endoscopic instruments in triangulation, and the ability to be rotated like human wrists. This is possible because of the seven degrees of freedom provided by the robotic wrists in the endoscopic end effectors [15].
We first performed an animal study in 2014 using two live porcine models to determine the feasibility of EFTR using the MASTER system [16]. In this study, we successfully performed two cases of EFTR with no injury to the surrounding structures throughout the procedure. We closed the gastric defect using Overstitch, with satisfactory gastric distention and no subsequent air leak. We demonstrated that the MASTER system affords superior maneuverability and triangulation compared to the currently available endoscopic instruments.
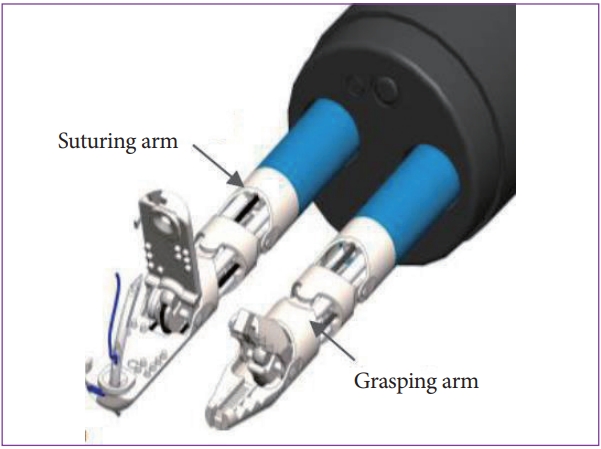
To ensure safe and reliable closure of the gastrointestinal tract defects, we developed a novel suturing device that can be used with the MASTER system in 2018. This novel suturing device consists of a needle driver and a grasper that can be inserted through-the-scope via the existing endoscopic channels (Fig. 7). Both robotic arms can be rotated up to 360 degrees. The needle driver has a locking mechanism, which allows the needles to be switched between its two jaws. An animal study performed using the MASTER system equipped with the suturing device showed that the use of a robot for endoscopic suturing is superior and effective for surgical suturing and creating the knot (Fig. 8) [17].
CONCLUSIONS
Given the limitations of ESD in the management of complex lesions, EFTR is especially useful for gastrointestinal tumors arising from deeper layers such as the submucosa or muscularis propria. EFTR is also beneficial for non-lifting lesions, and lesions located at difficult to access anatomical locations.
Many techniques have been developed to allow endoscopists to perform EFTR. However, despite multiple techniques, EFTR has not been widely adopted in routine clinical practice. This is due to the technical challenges faced by endoscopists using the currently available endoscopic devices. The lack of maneuverability and triangulation makes it technically demanding for tumor resection to be performed endoscopically. Moreover, the inability to secure reliable wound closure with the current available systems continues to be a challenge for endoscopists.
We have since developed a robotic endoscopic platform with suturing capabilities. Through our studies, we have demonstrated that the robotic endoscopic platform with suturing capabilities offers a promising way to overcome many limitations of the current endoscopic techniques used in EFTR. With the benefits offered by this system and its ease of use in the clinical setting, we believe that more endoscopists would adopt EFTR in clinical practice in the future.