Roles of Capsule Endoscopy and Device-Assisted Enteroscopy in the Diagnosis and Treatment of Small-Bowel Tumors
Article information
Abstract
With the development of capsule endoscopy (CE) and device-assisted enteroscopy (DAE), the incidence of small-bowel tumors has increased and the characteristics of these tumors have changed. In addition, the diagnostic and therapeutic approaches for small-bowel tumors have diversified. CE is a simple, noninvasive method that aid in the visualization the entire small bowel. CE is considered the initial approach for small-bowel tumors. DAE can be used to perform endoscopic procedures such as bleeding control, polypectomy, stent insertion, and tattooing, as well as for diagnosis through visualization or tissue sampling. Therapeutic intervention with DAE is particularly useful in polyposis syndromes such as familial adenomatous polyposis and Peutz-Jeghers syndrome. This review will discuss the roles of CE and DAE in the diagnosis and treatment of small-bowel tumors.
INTRODUCTION
Small-bowel cancer is a rare disease that accounts for <3% of all gastrointestinal (GI) malignancies although the small bowel makes up 75% of the length of the entire GI tract [1]. Previous studies have suggested that the reasons for this rarity include the rapid transit time with rapid epithelial turnover, alkaline environment, fluidity, low bacterial count, and high level of immunoglobulin A in the small bowel [2,3]. In addition, the small bowel is difficult to evaluate owing to poor accessibility because of its very long length (6–7 m) and the lack of a satisfactory diagnostic imaging modality [4].
The development of capsule endoscopy (CE) and device-assisted enteroscopy (DAE) has helped in the internal visualization of the small bowel, which was considered to be a “blind area”, and DAE has made it possible to perform endoscopic treatment as well as diagnosis [5]. Especially, CE is a simple and noninvasive method of taking images of the small-bowel lumen by swallowing a small capsule-type digital camera. Although DAE is a slightly more invasive method than CE, DAE can be used to obtain tissues for histologic diagnosis and to perform therapeutic interventions such as polypectomy, tattooing, balloon dilatation, and stent insertion [1].
Since the introduction of CE and DAE, the incidence of small-bowel cancer has increased. The incidence of small-bowel cancer in the United States, calculated using the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program 9 Incidence database, increased from 11.8 cases per million in 1973 to 22.7 cases per million in 2004 [6]. In Korea, the incidence of small-bowel cancer calculated using the Korea Central Cancer Registry nearly doubled from 0.8 cases per 100,000 in 1999 to 1.8 cases per 100,000 in 2017.
Most small-bowel tumors are clinically silent or manifest with nonspecific and vague symptoms. Therefore, small-bowel tumors are often accidentally diagnosed in the course of a diagnostic workup in patients with obscure GI bleeding (OGIB), unexplained iron deficiency anemia, unexplained abdominal pain, nausea and/or vomiting, or abnormal weight loss [2]. OGIB is the most common presentation of small-bowel tumors; however, severe bleeding is less common. Obstruction is also a common presentation. Consequently, nausea, vomiting, and recurrent crampy abdominal pain can occur. Unexplained weight loss is present in 30%–50% of patients with small-bowel tumors and requires a thorough investigation, especially in the elderly [2]. These features can delay the diagnosis and optimal treatment of small-bowel tumors.
This review will discuss the roles of CE and DAE in the diagnosis and treatment of small-bowel tumors.
SMALL-BOWEL TUMORS
Small-bowel tumors can be classified as malignant (adenocarcinoma, neuroendocrine tumor, lymphoma, sarcoma, etc.) or benign (adenoma, leiomyoma, lipoma, fibroma, hemangioma, etc.) [7]. Malignant small-bowel tumors are categorized into primary and secondary tumors. Benign tumors are more common than malignant tumors, accounting for up to 75% of all small-bowel tumors in an autopsy series [8]. The median age at diagnosis of a small-bowel neoplasm is in the sixth decade of life, with sarcoma and lymphoma presenting at a slightly younger age (early 60s) than adenocarcinoma and neuroendocrine tumors (late 60s) [9-11]. A slight male predominance (maleto-female ratio of 1.39–2.20) has been reported [11-13].
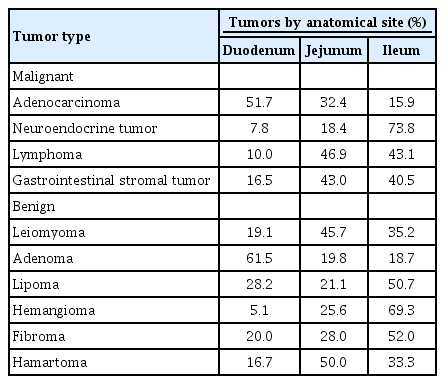
Adenocarcinoma has been the most common histologic type among small-bowel malignancies (accounting for 30%–50% of all primary malignant small-bowel neoplasms), followed by neuroendocrine tumor (20%–30%), lymphoma (16%), and sarcoma (10%) [12,14]. However, in recent years, the incidence of neuroendocrine tumors remarkably increased, being similar to or higher than that of adenocarcinoma [14]. The distribution of tumors by histologic type greatly varies according to location (Table 1) [9,11]. Adenocarcinomas are most frequently diagnosed in the duodenum, whereas neuroendocrine tumors are most frequently found in the ileum [14,15].
Secondary small-bowel cancer has been reported to be more frequent than primary small-bowel cancer. It occurs either by direct invasion from adjacent organs (such as the stomach, pancreas, liver, kidney, ovaries, or mesentery) or by distant metastasis. The most common primary origins of metastatic tumors of the small bowel are melanoma, lung cancer, breast cancer, cervical cancer, sarcoma, and colon cancer [8].
Small-bowel cancer has been reported to be associated with several medical and genetic predisposing conditions, such as Crohn’s disease, celiac diseases, familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer, Peutz-Jeghers syndrome (PJS), multiple endocrine neoplasia syndrome type 1, and neurofibromatosis type 1 [13,14].
The diagnosis of small-bowel tumors is difficult. Therefore, in patients suspected of having a small-bowel tumor, a diagnostic workup with a combination of laboratory, radiologic, and endoscopic examinations should be performed (Table 2) [1,8].
DIAGNOSIS USING CAPSULE ENDOSCOPY AND DEVICE-ASSISTED ENTEROSCOPY
Capsule endoscopy
CE is a noninvasive method for visualizing the whole small bowel. Therefore, CE is generally considered the initial approach for small-bowel tumors. However, patients with small-bowel tumors do not have definite signs and symptoms suggestive of the presence of the tumors. Small-bowel tumors are often accidentally diagnosed during the investigation of patients with OGIB, unexplained iron deficiency anemia, and unexplained abdominal pain after a normal endoscopic evaluation of the upper and lower GI tract [16].
The detection rate of small-bowel tumors by CE has been reported to range from 2% to 9% [1,17-20]. The main indication of CE in patients diagnosed with small-bowel tumors is OGIB [1,18-20]. In a European multicenter study that analyzed 5,129 patients undergoing CE, 2.4% (124 patients) were diagnosed with small-bowel tumors [19]. Among 124 patients with small-bowel tumors, 87.1% (108 patients) were examined for OGIB, 7.3% (9 patients) for abdominal pain, and 4.8% (6 patients) for the evaluation of a primary neoplasm. In a Korean multicenter study including 1,332 CE cases, 57 patients (4.3%) were diagnosed with small-bowel tumors (9 patients with genetic polyposis syndrome were excluded) [18]. In addition, patients with OGIB accounted for 75.4% (n=43), those with abdominal pain accounted for 14% (n=8), and those with abnormal weight loss accounted for 3.5% (n=2). Although most patients with small-bowel tumors diagnosed using CE underwent CE for OGIB, the incidence of small-bowel tumors in these patients ranged from only 0.35% to 2.3% [17,21].
CE is also useful for the surveillance of patients with hereditary polyposis syndrome. Intestinal polyposis syndromes can be divided into adenomatous polyposis, hamartomatous polyposis, and other rare polyposis [22].
During CE, small-bowel polyps in the jejunum or ileum were detected in 30%–60% of patients with FAP [22-25]. The detection rate of jejuno-ileal polyps was higher in patients with FAP with duodenal polyps than in those without duodenal polyps [23-25]. In patients with PJS, polyps most commonly occurred in the jejunum and ileum, and the detection rate of CE for small-bowel polyps ranged from 75% to 90% [24,25]. CE showed a better diagnostic yield for the detection of smaller jejuno-ileal polyps than other imaging modalities [2,22,26]. In a study that compared CE with magnetic resonance enterography, CE tended to miss large polyps more often than magnetic resonance enterography [27]. In another study, CE showed similar detection rates to DAE for small-bowel polyps in patients with PJS [26].
CE may easily miss tumors located in the duodenum or proximal jejunum because of rapid transit [2]. Endoscopy is usually reported to be a superior method for detecting duodenal polyps. In a pooled analysis evaluating the results of CE, the miss rate of small-bowel tumors was reported to be 18.9% (20/106 tumors) [28]. In a study including 300 patients who underwent CE for OGIB, CE missed 2 of the 10 confirmed small-bowel masses [17]. The tumors were located in the periampullary area and proximal jejunum. Therefore, additional radiologic examinations should be considered in the case of suspected small-bowel tumors.
The important consideration when performing CE is capsule retention. Fortunately, capsule retention rarely occurs and is usually not a serious complication. The rate of capsule retention varies depending on the indication and is particularly high at about 20% in cases of suspected small-bowel obstruction (1.5%–2.6% in the absence of obstructive symptoms) [17,29,30]. Therefore, patients with obstructive symptoms need more strict follow-up.
Device-assisted enteroscopy
DAE is a relatively noninvasive method. However, it is an uncomfortable procedure for patients, and is labor intensive and time consuming for the operator. While CE is usually considered the initial approach in the diagnostic workup for patients with suspected small-bowel diseases, DAE is useful for differential diagnosis or for confirming the diagnosis of lesions identified using CE or other imaging modalities through visualization or tissue sampling [2,31]. In addition, DAE can be performed in patients with obstructive lesions and offers endoscopic treatment such as balloon dilatation and stent insertion.
The diagnostic yield of DAE for small-bowel tumors has been reported to be 8.4%–16.8%, and it is generally comparable to that of CE [2,31-34]. However, the diagnostic yield of DAE for small-bowel tumors varies according to clinical indication and the higher small-bowel tumor detection rate of DAE has been suggested to result from the difference in the enrolled patients, such as those with suspected small-bowel lesions on previous radiologic studies or CE [2].
In a Japanese study that enrolled 1,035 patients who underwent DAE, small-bowel tumors were detected in 13.9% (144 patients) [32]. Among 144 patients with small-bowel tumors, the most common indication for DAE was OGIB (458 cases, 44.3%) and patients suspected of having small-bowel tumors comprised 9.0% (93 patients). However, the detection rate of small-bowel tumors was 8.5% in patients with OGIB (39/144 cases) and 65.6% in patients with suspected small-bowel tumors (61/93 cases). In another Chinese study of 2806 patients who underwent DAE, the detection rate of small-bowel tumors was 8.4% (236 cases) [31]. That study enrolled patients without a clear diagnosis in previous examinations including esophagogastroscopy, colonoscopy, abdominal computed tomography, and/or magnetic resonance imaging. In this study, the most common indication for DAE was abdominal pain (930 cases, 33.2%). Other cases were performed for weight loss in 28.3% (795 cases), OGIB in 20% (562 cases), diarrhea in 16.3% (457 cases), and obscure partial intestinal obstruction in 2.2% (62 cases). However, the detection rate of small-bowel tumors was highest in patients with OGIB (177/562 cases, 31.5%), followed by patients with obscure partial GI obstruction (10/62 cases, 16.1%).
In addition, DAE can detect lesions that were missed during CE. In a study including 15 small-bowel mass lesions in patients with OGIB who underwent both DAE and CE, CE identified 12 meaningful lesions as mass lesions in five patients and according to the presence of fresh luminal blood in seven patients, whereas DAE detected all 15 small-bowel mass lesions [35].
On the contrary, in another study that enrolled 78 patients with small-bowel tumors, DAE detected 67 tumors among 400 patients who underwent DAE (16.8%) [3]. The other 11 small-bowel tumors missed in DAE were diagnosed by surgery or CE. The reasons for the missed diagnoses during DAE were inadequate insertion depth in five cases, inappropriate insertion approach in four cases, and exophytic growing mass in two cases.
The insertion depth in DAE can vary depending on physician experience, patient characteristics (previous abdominal surgery, large body habitus, etc.), use of carbon dioxide insufflation, different methods such as double-balloon enteroscopy or single-balloon enteroscopy, and long procedure duration [1,8].
APPLICATION OF CAPSULE ENDOSCOPY AND DEVICE-ASSISTED ENTEROSCOPY FOR THE MANAGEMENT OF THE SMALL-BOWEL TUMORS
Although CE is useful as the initial approach for the diagnosis of suspected small-bowel lesions, it can also play various roles besides the diagnosis of small-bowel tumors. For example, CE can determine the extent of tumor involvement and monitor the response to treatment [1]. Further, CE can help decide the initial approach route for the DAE procedure. However, CE cannot be used to perform direct therapeutic interventions for small-bowel tumors.
On the contrary, DAE can be useful not only for the detection with histologic diagnosis of small-bowel tumors but also as a therapeutic intervention. The therapeutic interventions that can be performed during DAE include bleeding control with argon plasma coagulation or clips, polypectomy, balloon dilatation for stenotic lesions, and stent insertion.
DAE is particularly useful in patients with polyposis syndrome. In patients with FAP and PJS, DAE can facilitate the removal of polyps during screening, allowing to avoid unnecessary surgery. In these cases, CE is mainly used for the surveillance of polyps (Fig. 1). However, in patients with obstructi on or severe intra-abdominal adhesions, surgical treatment or intraoperative enteroscopy is required. PJS is one of the most common indications for enteroscopic polypectomy with DAE. To prevent complications from enteroscopic polypectomy, such as bleeding, perforation, and postpolypectomy coagulation syndrome, submucosal injection before polypectomy is generally recommended because the small bowel has a relatively thin wall [36]. Removal of polyps >15 mm is recommended because they could present adenomatous change and cause intussusception [36,37]. Therefore, polyps at least 10 mm in size should be resected as much as possible [36].

Surveillance and polypectomy using capsule endoscopy and device-assisted enteroscopy in a patient with Peutz-Jeghers syndrome. (A, B) Surveillance capsule endoscopy images showing several polyps >10 mm in the jejunum. (C-E) Enteroscopic polypectomy using device-assisted enteroscopy.
Stent insertion for malignant small-bowel obstruction can be performed using DAE. However, the working channel of the DAE instrument is small for inserting the stent device, and it is impossible to insert the stent using the through-the-scope deployment method. In a small case series, a self-expandable metallic stent was inserted for malignant small-bowel obstruction using a colonoscope in one patient and using the throughthe-overtube technique in two patients [38]. In a Korean study of 19 patients with small-bowel obstruction due to metastatic cancer, technical success was achieved in 18 cases (94.7%) in which a conventional endoscope was used after passing a guidewire using an enteroscope with or without ballooning [39]. However, stent insertion for malignant small-bowel obstruction can be impossible if the lesion is located in the deep small bowel, which is difficult to reach using DAE.
Tattooing in small-bowel tumors before surgery is another therapeutic advantage of DAE. This method is helpful for surgeons in performing the most appropriate surgical method and facilitating the localization of the small-bowel tumor during surgery [37,40]. In a study that analyzed 81 patients who received an ink tattoo during DAE, 10 patients underwent surgery [40]. All tattooed lesions were recognized, facilitating the localization of the target lesions during surgery.
CONCLUSIONS
Both CE and DAE are useful methods for diagnosing small-bowel tumors. CE is considered the initial approach for small-bowel tumors because it is noninvasive and can aid in the visualization of the entire small bowel. DAE is useful for differential diagnosis through visualization or histologic diagnosis of lesions identified using other imaging modalities. DAE can be used to perform therapeutic interventions such as polypectomy, stent insertion, and tattooing for small-bowel tumors. It is particularly useful in patients with polyposis syndrome. Patients with polyposis syndrome can avoid unnecessary surgery by undergoing polypectomy during DAE. Stent insertion and tattooing for small-bowel tumors using DAE are among the therapeutic options; however, it is important to select the appropriate indications. Although CE cannot be used in direct therapeutic interventions, it can help determine the extent of tumor involvement and monitor the response to treatment.
Notes
Conflicts of Interest: The author has no financial conflicts of interest.