AbstractBackground/AimsEndoscopic resection (ER) for superficial non-ampullary duodenal epithelial tumors (SNADETs) is challenging. Conventional endoscopic mucosal resection (CEMR) is also problematic due to the anatomical features of the duodenum. We compared the safety and efficacy of underwater endoscopic mucosal resection (UEMR) with those of CEMR through a retrospective analysis.
MethodsAltogether, 44 consecutive patients with 46 SNADETs underwent ER (18 CEMR cases and 28 UEMR cases) between January 2016 and October 2019. We investigated the proportions of en bloc resection, R0 resection, complications, resection time, and total procedure time and compared the outcomes of patients from the CEMR group with those of patients from the UEMR group.
ResultsThe median tumor size was 8.0 mm (range, 2.0ŌĆō20.0 mm). The UEMR group showed a higher proportion of en bloc resection (96.4% vs. 72.2%, p<0.05) and significantly lower median resection time and total procedure time (4 min vs. 9.5 min, p<0.05 and 13 min vs. 19 min, p<0.05; respectively) than the CEMR group. No complications were observed. However, two patients treated with piecemeal resection in the CEMR group had residual tumors.
INTRODUCTIONUntil recently, superficial non-ampullary duodenal epithelial tumor (SNADET) was considered a very rare disease, with estimated prevalence rate of 0.01%ŌĆō0.4% [1-4]. However, due to advances in endoscopic technology, detection rate of SNADET is increasing [5]. Non-ampullary duodenal adenomas are precancerous lesions and therefore, require early therapeutic intervention [6,7]. With minimal lymph node metastasis, less invasive endoscopic treatment is ideal for SNADETs [8-10]. However, standard endoscopic resection (ER) has not been established as a treatment for SNADETs due to a considerable rate of adverse events. Conventional endoscopic mucosal resection (CEMR) is the most common treatment for SNADETs. However, CEMR for SNADETs has a high recurrence rate (5%ŌĆō37%), with constant rates of adverse events such as delayed bleeding (0%ŌĆō15%), intraprocedural perforation (0%ŌĆō2%), and delayed perforation (0%ŌĆō4%) [10-14]. Endoscopic submucosal dissection (ESD) is widely accepted as a high-quality treatment for superficial neoplasias of the gastrointestinal tract regardless of the lesion size. Duodenal ESD has been associated with a greater number of adverse events such as perforation and bleeding [15-18]. Underwater endoscopic mucosal resection (UEMR) eliminates the need for submucosal injection. It has been attracting attention as an excellent endoscopic treatment for SNADETs with very few adverse events [19,20]. The present study aimed to investigate the preferred endoscopic treatment (UEMR or CEMR) for SNADET.
MATERIALS AND METHODSStudy designThe present retrospective analysis of ER for duodenal tumors was conducted at the Department of Gastroenterology of Nara Medical University (Nara, Japan). The study protocol adhered to the ethical principles of the Declaration of Helsinki and was approved by the institutional review board of Nara Medical University Hospital (approval no. 2049). All authors had access to the study data and approved the final manuscript. This manuscript is presented according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
PatientsConsecutive patients with duodenal tumors sized Ōēż20 mm who underwent ER between January 2016 and October 2019 were enrolled in this study. Patients with neuroendocrine tumors or ampullary duodenal tumors were excluded. PatientsŌĆÖ data and the details of the procedures were retrieved from the hospital database and from the patientsŌĆÖ charts. In our department, CEMR (the ŌĆ£injection and snaring methodŌĆØ or the ŌĆ£two-channel strip biopsy methodŌĆØ) was performed from January 2016 to January 2018 and UEMR was performed from February 2018 to October 2019. We evaluated patient characteristics, endoscopic findings, treatment outcomes including histological findings, and adverse events between patients who underwent CEMR and those who underwent UEMR.
ProceduresAll ER procedures were performed by board certified endoscopists of the Japan Gastroenterological Endoscopy Society with sufficient endoscopic treatment experience. CEMR was performed using either the ŌĆ£injection and snaring methodŌĆØ or the ŌĆ£two-channel strip biopsy methodŌĆØ. The ŌĆ£injection and snaring methodŌĆØ was performed principally using a therapeutic endoscope with a water jet function (GIF-Q260J; Olympus, Tokyo, Japan) and the ŌĆ£two-channel strip biopsy methodŌĆØ was performed using a two-channel endoscope (2T240; Olympus). For all EMR cases, a transparent cap (model D-201-11804 or D-201-13404; Olympus) was attached to the tip of the endoscope. Hypertonic saline-epinephrine or 0.4% sodium hyaluronate (Mucoup; Boston Scientific Japan, Tokyo, Japan) was injected into the submucosal layer before the initiation of both methods. Mucosal resection was performed using an electrocautery snare (Captivator II; Boston Scientific, Marlborough, MA, USA) powered by an electrosurgical generator (VIO300D; Erbe Elektromedizin, Tubingen, Germany). During the ŌĆ£injection and snaringŌĆØ process for CEMR, the lesion elevated with a submucosal injection was captured with an electrosurgical snare and resected with electrocautery (Endo Cut Q mode, effect 3, duration 2, and interval 4). During the ŌĆ£two-channel strip biopsyŌĆØ, a grasping forceps was inserted into the right channel of a two-channel endoscope and passed through an electrosurgical snare inserted from the left channel. The lesion elevated with a submucosal injection was grasped and pulled up with the forceps. The lesion was then captured and resected using an electrosurgical snare with electrocautery, similar to the ŌĆ£injection and snaring methodŌĆØ. UEMR was performed using a therapeutic endoscope with a water jet function (GIFQ260J; Olympus). For all UEMR cases, a transparent cap (model D-201-11804; Olympus) was attached to the tip of the endoscope. After complete deflation of the gastric and the duodenal lumens, 0.9% saline solution was infused until the lesion was adequately visualized in the saline. Three electrosurgical snare sizes were used according to the lesion size. Once the lesion was trapped, it was transected with electrocautery (Endo Cut Q mode, effect 3, duration 2, and interval 4) using the same electrosurgical generator used for CEMR. After resection with both the methods, any active hemorrhage was coagulated with hemostatic forceps (Coagrasper, FD410LR; Olympus) using the soft coagulation mode (effect 5, 80 W). Subsequently, the mucosal defect was observed carefully and additional resection was performed using the electrosurgical snare if an endoscopically apparent or suspicious residual tumor was observed. The surgeon attempted to close the mucosal defects with endoclips (EZ clip; Olympus) as frequently as possible to prevent delayed adverse events. Patients fasted for 2 days including the day of the resection procedure. After confirming that there were no adverse events, patients were allowed to begin a liquid diet on postoperative day (POD) 3 and were usually discharged on POD 6. Patients were administered proton pump inhibitors (lansoplazole 30 mg/day or esomeprazole 20 mg/day) or a potassium-competitive acid blocker (vonoprazan 20 mg/day) for 6 weeks. Resected specimens were embedded in 10% formalin and fixed specimens were sectioned serially at 2 mm intervals. Two experienced pathologists assessed the specimens according to the revised Vienna classification [21]. All patients underwent endoscopy at our hospital to assess any residual tumors 2 months after the procedure.
Data analysis and outcomesCollected data included patientsŌĆÖ age, sex, lesion characteristics (location, size, macroscopic type), resection time, total procedure time, en bloc resection rate, R0 resection rate, pathological diagnosis, adverse events, and recurrence. The resection time was measured from submucosal injection to lesion removal in CEMR and from underwater immersion to lesion removal in UEMR. Total procedure time was defined as the total time required for resection and closing the postER mucosal defect. R0 resection was pathologically defined as no tumor involvement up to the resection margins. Delayed bleeding was defined as hematemesis or melena requiring endoscopic hemostasis after the procedures. Perforation was classified into intraoperative and delayed perforation. The former was defined as a perforation that occurred during the procedure, while the latter was defined as any perforation observed thereafter.
Statistical analysisAll variables were presented as mean┬▒standard deviation or median (range). Chi-squared test and FisherŌĆÖs exact test or MannŌĆōWhitney U test were used to compare the baseline characteristics and measurements. All statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Co., Armonk, NY, USA) and a p-value <0.05 was considered statistically significant.
RESULTSCharacteristics of the patients and the lesionsIn the present study, 44 consecutive patients with 46 SNADETs underwent ER (18 cases treated with CEMR and 28 treated with UEMR). The median lesion size was 8.0 mm (2.0ŌĆō20.0 mm). Macroscopic evaluation revealed 36 elevated lesions (78.3%) and 10 depressed lesions (21.7%). The overall characteristics of the lesions included in this study are presented in Table 1. No significant differences were observed in lesion position, macroscopic type, and lesion size between the CEMR group and the UEMR group. The ŌĆ£injection and snaring methodŌĆØ was applied in six cases and the ŌĆ£two-channel strip biopsy methodŌĆØ was applied in 12 cases.
Histopathological resultsHistopathologically, the tumors included 19 adenomas/low-grade intraepithelial neoplasias (Category 3), 26 intramucosal adenocarcinomas/high-grade intraepithelial neoplasias (Category 4), and one submucosal invasive adenocarcinoma (Category 5). The accuracy of biopsy diagnosis was 63.0% (29/46) and the positive predictive value of the biopsy for Category 3 and Category 4 tumors according to the Vienna classification was 63.2% (12/19) and 63.2% (17/26), respectively.
Endoscopic treatment and complicationsThe treatment outcomes of the resected SNADETs are summarized in Table 2. The proportions of en bloc resection and R0 resection among all cases were 87.0% and 63.0%, respectively. The proportion of en bloc resection was significantly higher in the UEMR group than in the CEMR group (96.4% vs. 72.2%, p<0.05). The proportions of R0 resection in the UEMR group and in the CEMR group were 71.4% and 50.0%, respectively. The vertical margin was estimated to be negative for all lesions treated with UEMR and for 16/18 (88.9 %) lesions treated with CEMR. The resection time and the total procedure time in the UEMR group were significantly shorter than those in the CEMR group (4 min vs. 9.5 min, p<0.01 and 13 min vs. 19 min, p<0.05; respectively).
Altogether, seven CEMR cases were treated with the ŌĆ£injection and snaring methodŌĆØ and 11 CEMR cases were treated with the ŌĆ£two-channel strip biopsy methodŌĆØ. The treatment results were compared between the groups. No significant differences were observed in the lesion size (8 mm vs. 12 mm, p=0.267), en bloc resection rate (71.4% vs. 72.7%, p=1.000), R0 resection rate (42.9% vs. 54.5%, p=1.000), resection time (6 min vs. 11 min, p=0.107), and total procedure time (13 min vs. 21 min, p=0.070) between the groups.
We also investigated the results of each treatment method according to the location, size, and morphology of SNADETs. The summarized data are presented in Table 3. No significant differences were observed in the baseline characteristics between the groups. The location was divided into the ŌĆ£first partŌĆØ (n=10) and the ŌĆ£second partŌĆØ (n=35). Regardless of the location, the resection time was significantly shorter in the UEMR group than that in the CEMR group (12 min vs. 5.5 min, p<0.05 and 10 min vs. 4 min, p<0.01 for the ŌĆ£first partŌĆØ and the ŌĆ£second partŌĆØ, respectively). In the ŌĆ£second partŌĆØ, the UEMR group had a significantly higher en bloc resection rate than the CEMR group (71.4% vs. 100%, p<0.05). The UEMR group showed a significantly shorter resection time (10 min vs. 4 min, p<0.05) and a significantly shorter total procedure time (19 min vs. 11 min, p<0.05) for SNADETs sized Ōēż10 mm. We also performed an investigation based on the morphology (elevated type or depressed type). The resection time was significantly shorter in the UEMR group than that in the CEMR group for both elevated (9 min vs. 6 min, p<0.05) and depressed (12 min vs. 4 min, p<0.05) types. The total procedure time was significantly shorter in the UEMR group than in the CEMR group for depressed type (14 min vs. 22 min, p<0.05).
The rates of prophylactic clipping in the UEMR group and in the CEMR group were 100% (28/28) and 94.4% (17/18), respectively. Table 4 shows the results of adverse events and follow-up outcomes. No complications (delayed bleeding and intraoperative or delayed perforation) were observed in any of the groups.
At the follow-up endoscopy, two patients treated with piecemeal CEMR were confirmed to have residual tumors. These patients underwent additional endoscopic treatment and no recurrence has been observed for 6 months.
DISCUSSIONUEMR was recently developed in USA and reports of its utilization are increasing in Japan. Currently, the degree of SNADET malignancy is difficult to evaluate correctly based on preoperative endoscopic findings or target biopsy [22]. In our study, the accuracy of biopsy-based diagnosis of SNADET was 63.0%, indicating that the diagnostic ability of target biopsy is insufficient. The use of en bloc resection is desirable to arrive at the exact pathological diagnosis of SNADET, which is remarkably rare compared to other gastrointestinal neoplasias.
Our study indicated that UEMR involved a significantly high proportion of en bloc resection for SNADET. CEMR for SNADET is often difficult due to the poor operability of lesions in the duodenal lumen and the fibrotic nature of the submucosal layer, which results in poor lifting following the submucosal injection. Moreover, inadequate injection sometimes makes snaring difficult, leading to piecemeal resection and intraoperative perforation. While performing CEMR, in addition to snaring the submucosal distended lesion, we were relatively successful in using the ŌĆ£lift and cut methodŌĆØ to pull up the lesion by grasping it with the forceps before snaring. However, the forceps was pulled up only at one point and the direction of the force was restricted due to the use of an endoscope. Consequently, we could not achieve a high proportion of en bloc resection with CEMR.
In contrast, after suctioning out air followed by injecting saline into the duodenal lumen, the duodenal lesion and the surrounding normal mucosa floated upward, lifting the surface of the lesion rather than a single point. Furthermore, tension in the duodenal mucosa is nearly nonexistent in the submerged condition. Therefore, the submerged mucosa was easier to grasp than the mucosa filled with air. Thus, we could easily snare the duodenal lesion in the underwater condition, achieving an excellent rate of en bloc resection with UEMR (Fig. 1).
Oka reported that en bloc resection for colorectal neoplasia reduced the local recurrence rate when compared with piecemeal resection [23]. Although the observation period was short, we found no cases of residual tumor in the UEMR group. However, two cases of residual tumor were found in the piecemeal CEMR group. Generally, the large number of piecemeal specimens makes it difficult to accurately determine the curability of the lesion regardless of the resection method used.
CEMR cases required a certain amount of time before adequate submucosal lifting and ER had to be performed with insufficient submucosal lifting for some patients. Filling the duodenal lumen with saline is a simple method that can be easily applied by injecting saline through the channel of the endoscope. This process creates a stable field similar to adequate submucosal lifting in the submerged condition, enabling endoscopic treatment. Hence, the resection time and the total procedure time for UEMR were shorter than those for CEMR in this study.
Binmoeller et al. reported that endoscopic ultrasonography in the underwater condition revealed a circular shape of the muscularis propria while maintaining native thickness [19]. Using the snaring procedure in this situation reduces the risk of grasping the muscle layer and facilitates conditions in which the lesion can be treated safely without intraoperative perforation. In addition, the underwater resection itself may reduce thermal damage to the duodenal wall.
It has been reported that complete closure of the post-resection wound prevents exposure to bile and pancreatic fluid and may also reduce postsurgical bleeding and delayed perforation [13,24,25]. Our study showed no significant difference in the success rate of prophylactic clipping between the UEMR group and the CEMR group. However, we could easily perform endoclip closure of the mucosal defect in all UEMR cases, since the surrounding mucosa after UEMR remained soft due to the lack of submucosal injection.
Kiguchi et al. reported the retrospective therapeutic results of UEMR and CEMR for SNADETs sized Ōēż20 mm [26]. The primary endpoint of the study was the resection rate without conversion to ESD. The conversion rate to ESD was significantly lower in UEMR than in CEMR. However, among the patients who underwent ER without conversion to ESD, UEMR cases had a significantly lower proportion of en bloc resection and R0 resection than CEMR cases [26]. In contrast, the primary endpoints of our study were en bloc resection rate and R0 resection rate of UEMR or CEMR. UEMR cases had a significantly higher proportion of en bloc resection and R0 resection than CEMR cases. No cases were converted to ESD in the present study. Particularly, UEMR showed remarkable usefulness for SNADETs of the ŌĆ£second partŌĆØ and SNADETs sized Ōēż10 mm regardless of the lesion morphology. Duodenal ESD is one of the most challenging endoscopic procedures to perform in the digestive tract. UEMR is considered highly advantageous as an ER procedure for SNADET, since immediate change in the treatment strategy of difficult EMR cases to ESD requires a very advanced endoscopic technique.
While performing UEMR for SNADETs of the ŌĆ£second partŌĆØ of the duodenum, the procedure might be difficult or rather time-consuming, as the water continues to go down without filling. In our experience, by evacuating the air in the stomach, the maneuverability of the endoscope is improved and it becomes easy to fill the second portion of the duodenum with water. Moreover, postural change is also useful in achieving good conditions for UEMR.
Our study has several limitations. It was a single-center retrospective study with a small number of patients. Moreover, the follow-up period was short. Hence, collection of long-term follow-up data is warranted.
In conclusion, UEMR is a safe and effective treatment for SNADET when compared with CEMR. Further large-scale multicenter studies are needed to confirm the effectiveness of UEMR.
NOTESAuthor Contributions
Conceptualization: Masanori Furukawa, Akira Mitoro
Data curation: MF, Hiroaki Takaya, Kosuke Kaji, Hideto Kawaratani, Tadashi Namisaki, Kei Moriya
Formal analysis: MF, Takemi Akahane, Junichi Yamao
Investigation: MF, AM
Methodology: MF, AM
Project administration: MF, AM
Resources: MF, Takahiro Ozutumi, Yukihisa Fujinaga, Keisuke Nakanishi, Koh Kitagawa, Soichiro Saikawa, Sinya Sato, Yasuhiko Sawada
Supervision: Hitoshi Yoshiji
Writing-original draft: MF
Writing-review&editing: MF, AM
AcknowledgmentsWe thank Dr. Yoji Takeuchi (Department of Gastrointestinal Oncology, Osaka International Cancer Institute, Osaka, Japan) for his insightful comments.
Fig.┬Ā1.Underwater endoscopic mucosal resection for a duodenal intramucosal adenocarcinoma sized 11 mm. (A) Upper gastrointestinal endoscopy revealed an elevated lesion in the inferior duodenal angle. (B, C) The lesion floated up and became easy to snare in the submerged condition. (D) Ulcer after underwater endoscopic mucosal resection. (E) Complete closure with endoclips (EZ clip; Olympus, Tokyo, Japan). 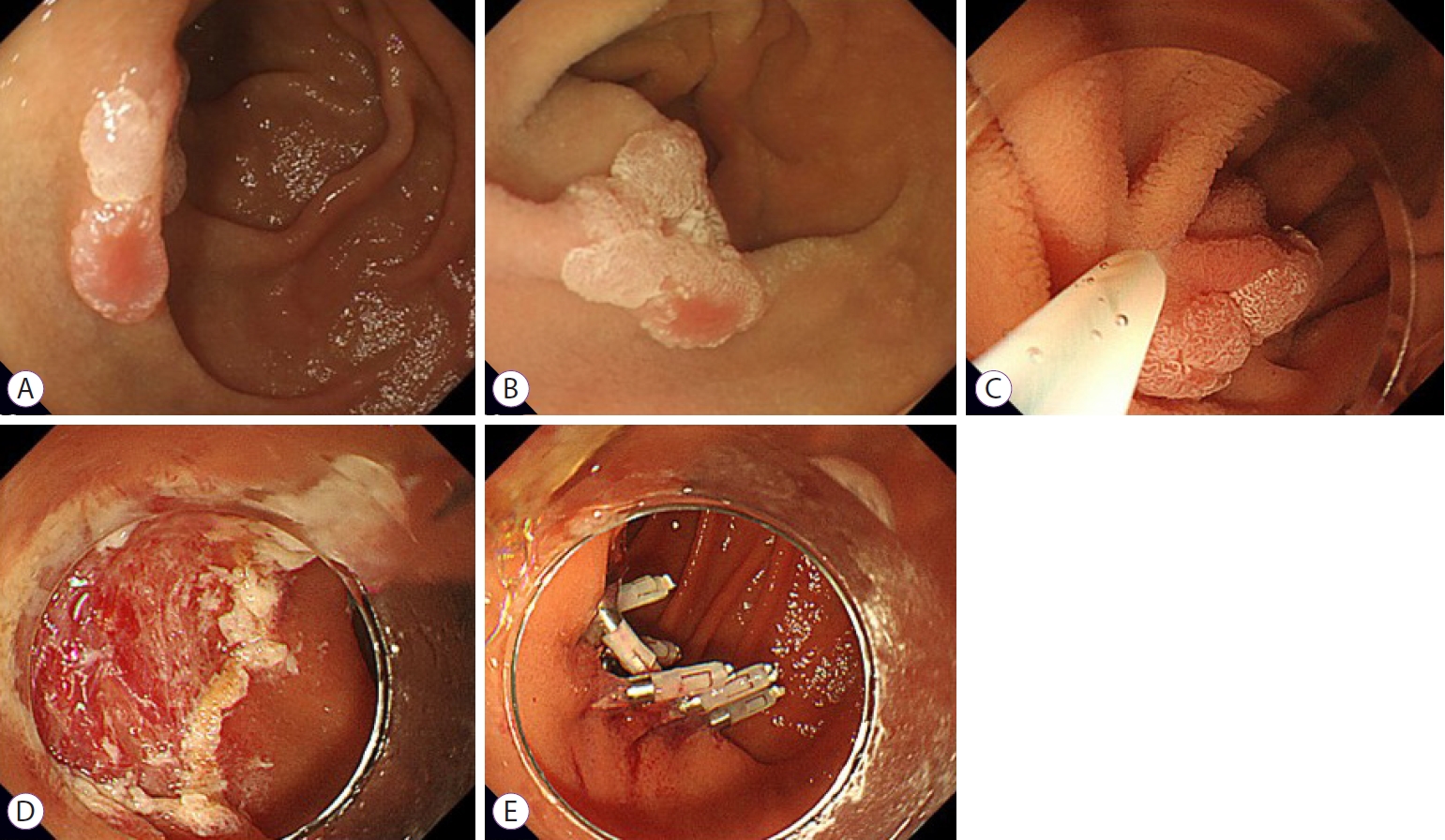
Table┬Ā1.Baseline Characteristics of Participants Table┬Ā2.Treatment Outcomes
Table┬Ā3.Detailed Outcomes of Each Endoscopic Resection Method
REFERENCES1. H├Čchter W, Weingart J, Seib HJ, Ottenjann R. [Duodenal polyps. Incidence, histologic substrate and significance]. Dtsch Med Wochenschr 1984;109:1183ŌĆō1186.
2. Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol 1994;29:483ŌĆō487.
3. Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut 2004;53:261ŌĆō265.
4. Jung SH, Chung WC, Kim EJ, et al. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol 2010;16:5474ŌĆō5480.
5. Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc 2014;26(Suppl 2):23ŌĆō29.
6. Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol 1992;87:37ŌĆō42.
7. Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol 2011;106:357ŌĆō364.
8. Asbun HJ. Management of duodenal polyps in the era of maximal interventional endoscopy and minimally invasive surgery: a surgical perspective. Gastrointest Endosc 2016;84:697ŌĆō699.
9. Friedrich-Rust M, Ell C. Early-stage small-bowel adenocarcinoma: a review of local endoscopic therapy. Endoscopy 2005;37:755ŌĆō759.
10. Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy 2015;47:129ŌĆō135.
11. Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy 2005;37:444ŌĆō448.
12. Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 2009;69:66ŌĆō73.
13. Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy 2013;45:138ŌĆō141.
14. Klein A, Nayyar D, Bahin FF, et al. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum: success, adverse events, and long-term outcomes. Gastrointest Endosc 2016;84:688ŌĆō696.
15. Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy 2013;45:133ŌĆō135.
16. Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy 2013;45:136ŌĆō137.
17. Hoteya S, Yahagi N, Iizuka T, et al. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum: feasibility and long-term outcomes. Endosc Int Open 2013;1:2ŌĆō7.
18. Inoue T, Uedo N, Yamashina T, et al. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc 2014;26:220ŌĆō227.
19. Binmoeller KF, Shah JN, Bhat YM, Kane SD. ŌĆ£UnderwaterŌĆØ EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest Endosc 2013;78:496ŌĆō502.
20. Yamasaki Y, Uedo N, Takeuchi Y, et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy 2018;50:154ŌĆō158.
22. Kinoshita S, Nishizawa T, Ochiai Y, et al. Accuracy of biopsy for the preoperative diagnosis of superficial nonampullary duodenal adenocarcinoma. Gastrointest Endosc 2017;86:329ŌĆō332.
23. Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015;110:697ŌĆō707.
24. Hoteya S, Kaise M, Iizuka T, et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc 2015;27:323ŌĆō330.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
