Does the Reprocessing of Endoscopes Have to Take Place Immediately after Pre-Cleaning? A First Evaluation
Article information
Abstract
Background/Aims
The recommendations on the time interval between pre-cleaning and reprocessing of endoscopes differ in international guidelines, with a low level of evidence. The aim of this study was to investigate the influence of postponing reprocessing on the reprocessing quality after pre-cleaning the flexible endoscopes.
Methods
We reprocessed 124 standardized test tubes simulating endoscope channels after soiling and contamination and determined the reprocessing performance. In addition, we examined contaminated gastroscopes, colonoscopes, and bronchoscopes. The duration of interim storage after pre-cleaning was 16 h for 100 test tubes and up to 24 h for 18 endoscopes. We determined the residual protein content and germ load as markers for cleaning and disinfection performance. In addition, we determined biofilm formation by photometry of crystal violet staining.
Results
All test tubes and flexible endoscopes showed residual protein content and germ load significantly below legally prescribed threshold values, independent of the interval between pre-cleaning and reprocessing.
Conclusions
Our findings indicate that flexible endoscopes could be stored overnight after pre-cleaning without any influence on the quality of reprocessing. While ensuring patient safety, this could simplify logistical processes and enable cost savings.
INTRODUCTION
The professional reprocessing of flexible endoscopes is essential to prevent infection and for patient safety purposes. There have been multiple outbreaks of multidrug-resistant organisms caused by inadequately reprocessed endoscopes [1-3]. However, special technical knowledge and considerable personnel resources through manual work steps are required to ensure the continuous quality of endoscope reprocessing [4,5]. In Germany, the joint recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) and the Federal Institute for Drugs and Medical Devices (BfArM) on the reprocessing criteria of flexible endoscopes and other medical devices is decisive [6]. It suggests the possibility of interim storage of medical devices during reprocessing. In the case of flexible endoscopes, a pre-cleaning or bedside cleaning process must be carried out immediately after use to remove coarse contaminants such as blood and tissue. The time interval of interim storage is not specified in the German recommendation. Recommendations of other European and Anglo-Saxon countries, including Korea, do not propose an interim storage time after pre-cleaning [7-14], or they propose short periods between 30 minutes and 3 hours [15-18]. The maximum possible time interval may be based on purely theoretical considerations [18-20]. Empirical studies on the effects of a postponement on the reprocessing quality are not available. Due to these vague time frames, practitioners sometimes use single-use endoscopes during weekends or night shifts when centralized reprocessing of regular endoscopes cannot be ensured within 3 h. These endoscopes, however, tend to be inferior in diagnostic purposes than regular endoscopes. In order to ensure consistent patient safety, this study examines the influence of interim storage on the reprocessing quality and evaluates whether time periods longer than 3 h would allow high quality reprocessing.
MATERIALS AND METHODS
To examine the reprocessing performance, we used standardized test tubes and real endoscopes that had already been applied to patients. We investigated the effects of the duration of the time interval after pre-cleaning on the reprocessing performance by determining the cleaning performance, disinfection performance, and biofilm content.
Test material
Based on a previous study, we used 124 test tubes that were produced and validated as test materials [21-23]. These were transparent polytetrafluoroethylene tubes with a length of 200 cm, an inner diameter of 2 mm, and a wall thickness of 0.5 mm (HYBETA GmbH; Münster, Germany). In addition, we examined 6 bronchoscopes, 6 gastroscopes, and 6 colonoscopes used clinically for at least 2 years (Olympus Deutschland GmbH; Hamburg, Germany).
Contamination
To investigate the cleaning performance, the test tubes were contaminated with a soiling solution of heparinized sheep blood and protamine in sodium dodecyl sulfate (SDS) according to method A in accordance with the Guideline for the Validation of Mechanical Cleaning and Disinfection Processes for the reprocessing of thermolabile endoscopes (see Supplementary Material 1 [S1]) [24]. Based on a previous study, we investigated the disinfection performance by introducing a defined quantity of a test organism into the test tubes with the contamination (E. faecium ≥109 Colony-forming unit (CFU)/mL) [23], aligned with the usual contamination of endoscopes after use, between >103 and 1010 CFU/mL (see S1) [25]. The contaminated test tubes are shown in Fig. 1.
The bronchoscopes, gastroscopes, and colonoscopes were not particularly contaminated since they were contaminated through usage in patients.
Pre-cleaning, storage and reprocessing
The tubes and endoscopes were prepared according to above-mentioned KRINKO and BfArM recommendations (Tables 1 and 2) [6]. In order to check all reprocessing protocols established in our hospital and to increase the external validity, the tubes were reprocessed at least five times at four locations.
The time interval of storage between pre-cleaning and subsequent reprocessing differed. In 100 cases, a duration of 16 hours was chosen, since it was the most clinically significant time interval if reprocessing takes place daily during core working hours, including weekends. In addition, storage times from 0 to 48 h were assessed. In endoscopes, the time intervals were 0, 6, and 24 h after pre-cleaning. The endoscopes were stored at room temperature.
Assessment of cleaning and disinfection performance
The cleaning and disinfection performance was examined according to the currently valid acceptance criteria in Germany, as they are used to evaluate reprocessing in the context of validation of mechanical cleaning and disinfection processes for reprocessing of thermolabile flexible endoscopes [22-24], and according to the KRINKO/BfArM recommendation [6]. These differ from the International Organization for Standardization (ISO)/Technical Specification (TS) 15883-5 by the reduced bacterial count [21]. In brief, this entails an optical cleanliness, a residual protein content ≤100 µg/test tube (cleaning performance), and a reduction factor of ≥9 log10/test tube (disinfection performance). For endoscopes, a residual protein content ≤100 µg (cleaning performance) and <10 CFU/10 mL, and no growth of Escherichia coli, Enterobacteriaceae, Enterococci, Pseudomonas aeruginosa, Pseudomonas spp., non-fermenters, and greening streptococci (disinfection performance) were used for acceptance.
Overall, 124 test tubes were reprocessed after the reprocessing protocols specified in Table 1. Subsequently, the test tubes were sent to the laboratory of HYBETA GmbH without further treatment, where the cleaning and disinfection performance was assessed. HYBETA used a bicinchoninic acid assay (BCA assay) to measure protein residues with a minimum detection of 30 µg/test object and 5 µg/endoscope and a maximum validated linear detection ability of up to 250 µg protein, according to German standards [6,22,23]. A 5-point calibration curve from 0 to 200 µg/mL was added to each 96 well plate as a positive control and to ensure precise linear detection. The correlation coefficient of calibration was at least 0.98.
In total, 18 endoscopes, notably 6 gastroscopes, 6 colonoscopes, and 6 bronchoscopes, were reprocessed after clinical use according to the protocol specified in Table 2. The duration of the time interval between pre-cleaning and subsequent reprocessing varied from 0 h to 24 h. After reprocessing, samples were taken from the instrument channel and additional irrigation channel and gastroscopes and colonoscopes were taken from the air/water channel, and from the suction channel (see Supplementary Material 2). To investigate the cleaning performance, the respective channel was rinsed four times with the same 10 mL of 1% SDS solution. To examine the disinfection performance, the channels were rinsed once with 20 mL of sterile physiological sodium chloride solution. The samples used to demonstrate the cleaning performance were examined in the laboratory of HYBETA GmbH by BCA assay. The samples assessed for the disinfection were examined in the Laboratory for Technical Hygiene at the Section for Hospital Hygiene and Environmental Health, Center for Infectious Diseases, Heidelberg University Hospital, as detailed in the Supplementary Material 3.
The cleaning and disinfection performance examinations were carried out separately for each endoscope to exclude any influence of the examination on the result.
Investigation of biofilm formation
Biofilm formation was identified using a validated method proposed by Günther et al. [26]. Therefore, three test tubes were soiled and contaminated as described above and reprocessed according to Table 1. The process was stopped (i) after pre-cleaning and 24-h storage, which served as a positive control, (ii) after pre-cleaning, 24-h storage, and brush cleaning, or (iii) after pre-cleaning, 24-h storage, and complete reprocessing. Subsequently, the biofilm was measured. An unsoiled test tube was used as a negative control. To investigate biofilm formation, the test tube was rinsed twice with 200 µL distilled water. The biofilm was stained by adding 150 µl of 0.1% crystal violet solution and stored for 20 min at room temperature. Afterwards, the test tubes were rinsed twice with 200 µl distilled water to remove excess crystal violet. Finally, the crystal violet dye bound in the biofilm was washed out by adding 150 µL of 10% ethanol. We determined the quantity of bound crystal violet in each test tube as a surrogate marker for the still existing biofilm mass six times by photometric absorption measurement at a wavelength of 570 nm.
RESULTS
Test tubes
In total, 124 soiled and soiled/contaminated test tubes were reprocessed after interim storage for 0–48 h after pre-cleaning (Fig. 2). All test tubes were optically clean regardless of the time interval and had a residual protein content of <30 µg/test tube (detection minimum). A reduction factor ≥9 log10/test tube was determined for the number of CFUs of E. faecium (Fig. 2). Thus, the cleaning and disinfection performance at extended storage intervals after pre-cleaning met the acceptance criteria.
Endoscopes
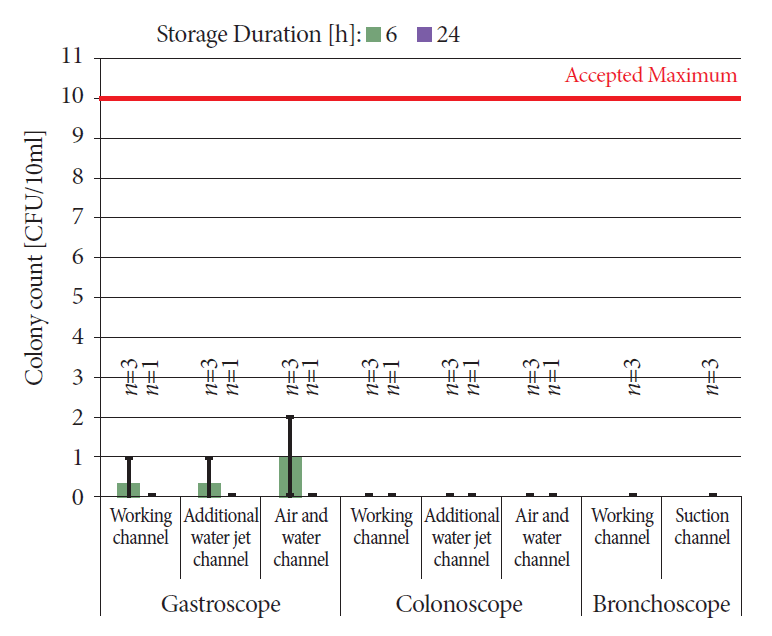
To increase the transferability to the clinical setting and account for differences in design and for the effects of material wear such as roughening in the canal lumen, the cleaning and disinfection performance was repeated on endoscopes used for at least 2 years. After usage, the 6 bronchoscopes, 6 gastroscopes and 6 colonoscopes were reprocessed and stored for up to 24 h after pre-cleaning. No endoscope was contaminated with a protein residue above the detection limit of 5 µg/endoscope. Therefore, the acceptance criterion of a protein residue of ≤100 µg was met in all the collected samples. Furthermore, we noted a colony count <10 CFU/10 mL (Fig. 3) and no growth of Escherichia coli, Enterobacteriaceae, Enterococci, Pseudomonas aeruginosa, Pseudomonas spp., non-fermenters, and greening streptococci. Thus, the acceptance criteria for both cleaning performance and disinfection performance were met.
Biofilm formation
We investigated biofilms formation at several points in the reprocessing protocol (Fig. 4). While biofilm was detected after pre-cleaning and 24-h storage, the optical density (OD) after pre-cleaning, storage, and brush cleaning, and after complete reprocessing with storage was similar to that of the uncontaminated control.

Photometric investigation of biofilm formation in the treatment process. Three test tubes were soiled and contaminated with Enterococcus faecium. During the preparation according to protocol 1, they were stored for 24 h after pre-cleaning. One test tube that was not soiled served as negative control. As a marker for biofilms, an optical density (OD) of 570 nm after staining with crystal violet was determined 6 x per test tube. Bars indicate standard deviation.
DISCUSSION
To our knowledge, this is the first study to examine the influence of prolonged storage time periods of flexible endoscopes after pre-cleaning on reprocessing quality. We examined the storage durations for up to 48 h in 124 test tubes and up to 24 h in 18 bronchoscopes, gastroscopes and colonoscopes. The cleaning and disinfection performance was orientated according to the German legislation and guidelines [6-24] and ISO/TS 15883-5 from 2005 [21]. This differed from the European norms by the more stringent acceptance criteria (lower threshold values for non-acceptance) and an additional patency test. All endoscopes and test tubes met the quality criteria for reprocessing, even after longer storage time periods.
We also used validated single-use test tubes to increase the size of the repetitions. The highest number of repetitions (n=50) was chosen for the 16-h interval, as this is probably the maximum storage period assuming reprocessing was carried out only during core working hours, including weekends.
As described at the beginning, the relevant period was not defined by official bodies in Germany [24]. The US-American Multisociety Guideline recommends cleaning and drying after pre-cleaning [8]. The Dutch Steering group for flexible endoscope cleaning and disinfection and the French Ministère des Affaires Sociales et de la Santé state that manual cleaning should take place immediately after pre-cleaning [10,11]. Similarly, the Swiss guidelines for reprocessing flexible endoscopes and the Australian Infection Control Guidelines of the Gastroenterological Society of Australia and the Australian Gastrointestinal Endoscopy Association require immediate pre-cleaning and manual cleaning after use [12,17]. This is referred to in the international paper of the European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology Nurses and Associates, which justifies the formulation of a maximum time interval of one hour with theoretical considerations on the population kinetics of microorganisms and the fixation of proteins by drying [19,20]. Specifically, it is assumed that the shorter doubling time of gram-negative bacteria of a minimum of 20–30 min allows the formation of biofilms if the entire treatment process is not carried out in a timely manner. This consideration does not consider the validity of this information for optimal growth conditions in vitro, that pre-cleaning directly after the patient examination can reduce contamination, and that an emerging biofilm can possibly be removed during reprocessing. Our experiment on biofilm formation suggests that biofilm can be formed during storage after pre-cleaning, but that it can be removed by brushing afterwards. This procedure of visualizing and quantifying cells adhering to the tube wall by crystal violet solution has been described by O’Toole and Kolter [27]. Biofilm production can be assumed if the OD is greater than the mean value of the negative control, added thrice to its standard deviation [28]. Although Stepanovic et al. [28] tested the biofilm of Staphylococci on microtiter plates, the basic consideration that justifies the above formula is justified and plausible; this is that biofilm production is subject to a variety of methodological influences.
Our study has some limitations. The number of repetitions is still too low to conclude sufficient preparation quality, especially for storage times other than 16 h. Therefore, we encourage researchers, especially those with different methodologies, to repeat the assay. Other flexible endoscopes were not assessed due to their critical reprocessing procedures (e.g. duodenoscopes) or because they are usually single-use products (e.g. ureteroscopes).
The recommended methods used to validate reprocessing for flexible endoscopes differ from country to country. For example, in contrast to the German/European norms, the US Food and Drug Administration (FDA) recommends the thresholds for an acceptable level of contamination of <6.4 µg/cm2 protein, <2.2 µg/cm2 hemoglobin, and <1.8 µg/cm2 carbohydrate [29,30]. Furthermore, the techniques of sampling strategy differ (brushing or simple flushing), volume, and used substances (saline, demineralized water, neutralizer) [31,32]. In addition, the contents of the test soil are discussed internationally. Some authors do not recommend heparin for the validation of flexible endoscope reprocessing, as it may inhibit some bacterial strains from adhering and from forming biofilms. They recommend using coagulated blood as test soil or preferably ATS2015 and Edinburgh-M soils [33,34]. The European Committee for Standardization informatively recently suggests the contamination of flexible endoscopes with biofilm-producing Pseudomonas aeruginosa for the examination of cleaning performance of washer disinfectors [35]. Therefore, it would be interesting to reevaluate our results by employing the newly suggested contamination method or international methods of contamination, sampling, and evaluation.
In summary, our findings may indicate that flexible endoscopes can be stored after pre-cleaning for up to 16 h without any influence on the reprocessing quality according to current test standards. We propose to evaluate international protocols and acceptance criteria for the reprocessing of flexible endoscopes also for longer storage periods if pre-cleaning is ensured. This would presumably reduce the use of single-use endoscopes, which would increase diagnostic quality and enable cost-savings.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: Martin Scherrer
Data curation: Vanessa M Eichel, MS
Formal analysis: VME, MS
Investigation: VME, MS
Methodology: VME, MS
Project administration: Samy Unser
Resources: Jonas M Jabs, SU
Supervision: Nico T Mutters, MS
Validation: NTM, MS
Visualization: VME, JMJ, SU, MS
Writing-original draft: VME
Writing-review&editing: NTM
Acknowledgements
We thank the staff of the interdisciplinary endoscopy center, the sterility supply departments of the orthopedic ward, the children’s clinic, and the head clinic of the Heidelberg University Hospital for most of the practical implementation and for their willingness to adapt the procedures to the needs of our study. We also thank Olympus Deutschland GmbH for the provision of loaner endoscopes and the companies Hybeta GmbH and Karl Storz SE & Co. KG.