Hematochezia in Patient with Rectal Tumor: Consideration of Various Diagnostic Possibilities
Article information
Quiz
A 74-year-old woman presented with hematochezia. The patient had bloody stool (approximately 50 cc) for the past 10 days. The patient had hypertension and was taking antihypertensive medications. The patient underwent total laparoscopic hysterectomy and bilateral salpingo-oophorectomy for endometrial cancer 2 years ago. After presentation, the patient underwent a colonoscopy. Approximately 3 cm mass was found near the anal canal (Fig. 1A). The mass was round and covered with exudate (Fig. 1B). Endoscopic ultrasonography showed a heterogeneous hypoechoic lesion located in the submucosal layer (Fig. 1C). Histopathological examination revealed small malignant oval-shaped cells with high nuclear-to-cytoplasmic ratio and poor differentiation (normal glandular structure was not observed) (Fig. 2). Computed tomography revealed a 3.5 cm enhancing lesion involving the distal rectum and anorectal junction (Fig. 3). Positron emission tomography revealed a mass of approximately 3-3.5 cm with increased FDG uptake (SUVmax =10.79) in the distal rectum (Fig. 4). Transanal excision was performed, and the resected lesion showed an ill-demarcated ulcerative and fungating mass, measuring 3.3 × 2.7 cm extending to the pericolic soft tissue (Fig. 5). In the final pathological examination, round cells with a high nuclear-to-cytoplasmic ratio without macronucleoli, which invaded the entire mucosal layer, were observed (Fig. 2, 6A and B). Immunohistochemistry for CD-3 and CD-20 was negative. Diffuse brown colored pigmentation was observed (Fig. 6C), and immunohistochemistry for HMB-45 and S-100 was positive (Fig. 7). What is the most probable diagnosis?
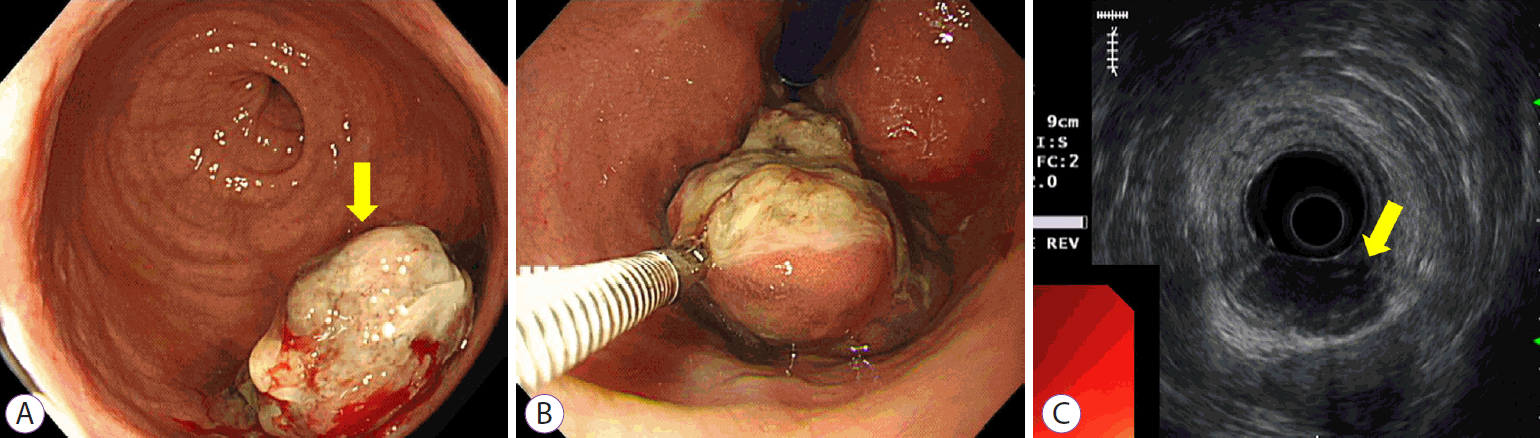
(A, B) Colonoscopy demonstrating 3 cm mass covered with exudates near anal canal. (C) Endoscopic ultrasonography demonstrating heterogenous hypoechoic lesion in the submucosal layer.
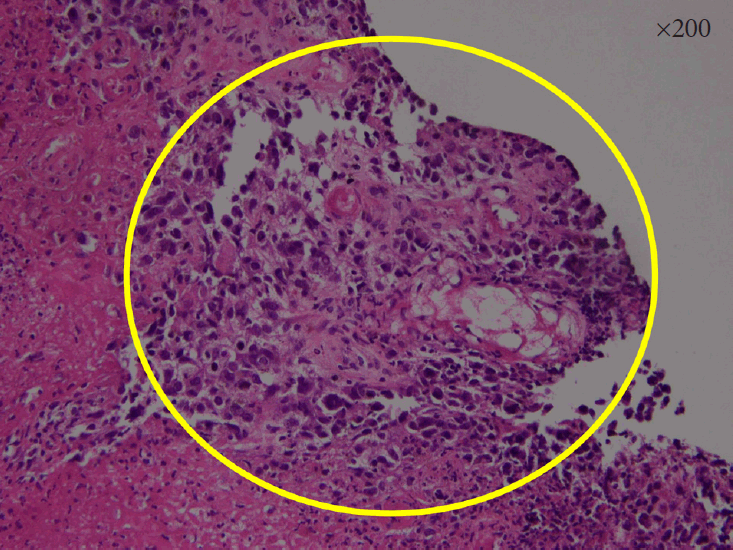
Histopathological findings of the biopsy specimens. Small malignant oval-shaped cells with high nuclear-to-cytoplasmic ratio with poor differentiation (normal glandular structure was not observed) (hematoxylin and eosin stain ×200).

(A, B) Contrast-enhanced computed tomography demonstrating 3.5 cm enhancing lesion involving distal rectum and anorectal junction.
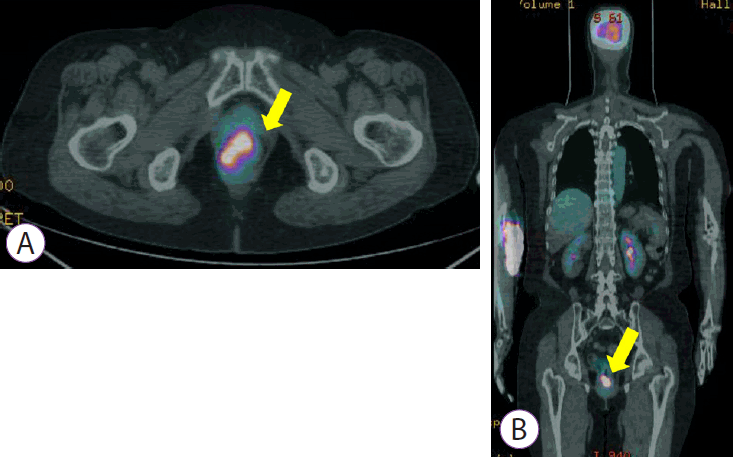
(A, B) Positron emission tomography demonstrating 3-3.5 cm mass with increased FDG uptake (SUVmax=10.79) in the distal rectum.
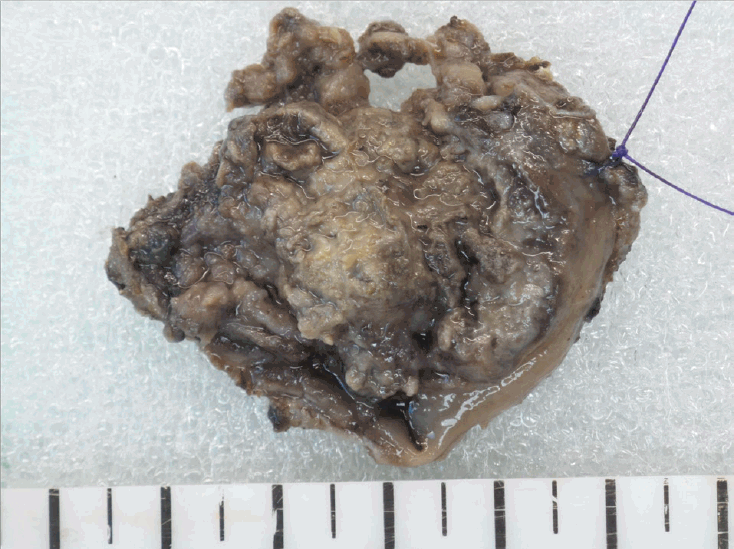
Surgical specimen demonstrates an ill demarcated ulcerative and fungating mass, measuring 3.3×2.7 cm extending to the pericolic soft tissue.
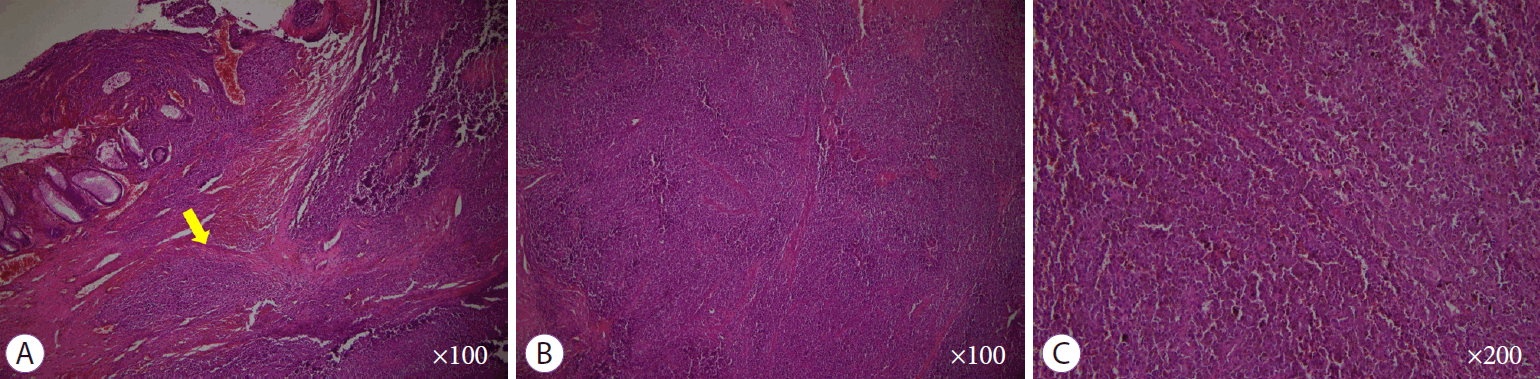
Histopathological findings of the surgically resected specimen. (A, B) Round cells with a high nuclear-to-cytoplasmic ratio without macronucleoli which invaded whole mucosal layer (hematoxylin and eosin stain ×100). (C) Diffuse brown colored pigmentation (hematoxylin and eosin stain ×200)
Answer
The patient was diagnosed with rectal melanoma. On colonoscopy, black pigmentation was not clearly observed on gross findings, and poorly differentiated adenocarcinoma was suspected based on the first histological examination. The mass was removed by transanal excision, and final immunohistochemistry for CK-20 (one of the markers for colorectal cancer) was negative, and for HMB-45 and S-100 was positive. Eventually, the patient was diagnosed with amelanotic melanoma. Primary anorectal melanoma is a rare tumor, accounting for only approximately 0.05% to 4.6% of all reported anorectal malignancies [1]. Anorectal lesions account for 0.3% of all melanomas [2]. Furthermore, approximately 30% of anorectal melanomas are amelanotic and can resemble benign polypoid lesions [3]. The most common symptom is hemorrhage [1], and patients diagnosed with anorectal malignant melanoma usually have locoregional metastasis [4]. Endoscopy or endoscopic ultrasound can be considered for diagnosis [5]. Immunohistochemistry is the cornerstone for the diagnosis of malignant melanoma. S-100, HMB-45, vimentin, and Melan A are important markers for the diagnosis of malignant melanoma. There is currently no standard treatment for anorectal amelanotic melanoma due to the low incidence and lack of evidence in the literature [1]. However, surgery in combination with immunotherapy, such as interferon, has been reported as a treatment option for localized tumors [6]. Chemotherapy and radiation therapy are known to play only a limited role [7]. Despite the recent treatment experience in Japan (successful treatment with nivolumab, an antibody to programmed death (PD-1) for controlling metastasis) [8], the overall treatment experience is still limited. Therefore, early diagnosis is important for the prognosis [9].
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.

