A multicenter comparative study of endoscopic ultrasound-guided fine-needle biopsy using a Franseen needle versus conventional endoscopic ultrasound-guided fine-needle aspiration to evaluate microsatellite instability in patients with unresectable pancreatic cancer
Article information
Abstract
Background/Aims
Immune checkpoint blockade has recently been reported to be effective in treating microsatellite instability (MSI)-high tumors. Therefore, sufficient sampling of histological specimens is necessary in cases of unresectable pancreatic cancer (UR-PC). This multicenter study investigated the efficacy of endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) using a Franseen needle for MSI evaluation in patients with UR-PC.
Methods
A total of 89 patients with UR-PC who underwent endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or EUS-FNB using 22-G needles at three hospitals in Japan (2018–2021) were enrolled. Fifty-six of these patients (FNB 23 and FNA 33) were followed up or evaluated for MSI. Patient characteristics, UR-PC data, and procedural outcomes were compared between patients who underwent EUS-FNB and those who underwent EUS-FNA.
Results
No significant difference in terms of sufficient tissue acquisition for histology was observed between patients who underwent EUS-FNB and those who underwent EUS-FNA. MSI evaluation was possible significantly more with tissue samples obtained using EUS-FNB than with tissue samples obtained using EUS-FNA (82.6% [19/23] vs. 45.5% [15/33], respectively; p<0.01). In the multivariate analysis, EUS-FNB was the only significant factor influencing the possibility of MSI evaluation.
Conclusions
EUS-FNB using a Franseen needle is desirable for ensuring sufficient tissue acquisition for MSI evaluation.
INTRODUCTION
Pancreatic cancer (PC) is a lethal disease and a major cause of cancer-related deaths worldwide.1 One reason for the poor prognosis associated with PC is that when most patients with PC are diagnosed, the tumor is already unresectable (UR)2-4; therefore, chemotherapy is the main treatment for unresectable PC (UR-PC) patients. According to the guidelines for treating PC published by the American Society of Clinical Oncology and the Japan Pancreatic Society, pembrolizumab is a second-line treatment for microsatellite instability (MSI)-high UR-PC.5,6 MSI is a predictive marker for the curative effect of immune checkpoint blockades. Recently, MSI evaluation using the Promega panel (Promega, Madison, WA, USA) has been covered by national health insurance in Japan. Therefore, it is desirable to obtain tissue samples from UR-PC patients for MSI evaluation.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an efficient and a safe method for sampling PC specimens.7-9 However, endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) using a Franseen needle has been reported more useful than EUS-FNA for tissue sampling.10,11 A previous report indicated that tissue sampling using EUS-FNB was useful for MSI evaluation in UR-PC patients.12 However, the previous study was performed at a single institution, and few UR-PC patients underwent both EUS-FNB and MSI evaluation. Therefore, whether EUS-FNB using a Franseen needle is useful for MSI evaluation in patients with UR-PC remains unknown. Therefore, we performed this multicenter study to determine the efficacy of EUS-FNB using a Franseen needle for sampling a sufficient amount of tissue for MSI evaluation.
METHODS
Patients
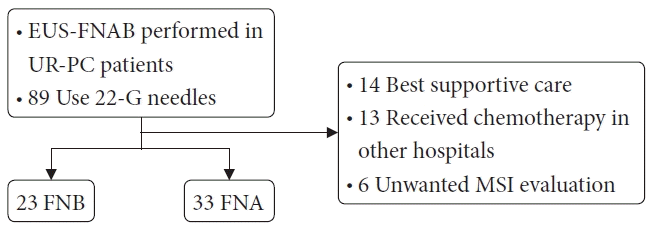
A total of 89 UR-PC patients who underwent EUS-FNA or EUS-FNB (EUS-FNAB) using 22-G needles between December 2018 and December 2021 at three hospitals in Japan (Fukushima Medical University Hospital, Aizu Medical Center of Fukushima Medical University, and Ohtanishinouchi Hospital) were enrolled (Fig. 1). Among these patients with UR-PC, 56 were followed up or evaluated for MSI. A total of 23 and 33 patients underwent EUS-FNB and EUS-FNA, respectively. In contrast, 33 patients were excluded because they were not evaluated for MSI: 14 patients were treated with best supportive care, 13 were treated with chemotherapy at other hospitals, and six chose not to undergo MSI evaluation. Thirteen patients who received treatment at other hospitals did not undergo subsequent MSI evaluation of the EUS-FNAB specimens.
Criteria for the three hospitals
All institutions that participated in this study were affiliated hospitals at the Fukushima Medical University. Therefore, the procedural and histological diagnostic criteria were almost identical. In addition, histological specimens obtained from the Ohtanishinouchi Hospital were sent for analysis to the Department of Diagnostic Pathology, Fukushima Medical University, through highly secure internet communication.
EUS-FNAB
After the patients were sufficiently sedated with intravenous administration of midazolam and placed in the left lateral decubitus position, we gently inserted an echoendoscope. When the lesion was observed on the monitor, the lack of blood flow on the puncture line was confirmed using doppler mode. The lesion was punctured and the stylet was removed. The needle was passed back and forth 20 times in the lesion while suction was applied using a 10-mL or 20-mL syringe. White lumbricoid specimens were separated from all the specimens collected using EUS-FNAB. The lumbricoid specimens were preserved in a bottle filled with formalin for subsequent histological diagnosis. The remaining specimens were used for cytology and rapid onsite evaluation (ROSE).13 Repeat aspiration was performed until a sufficient specimen was obtained, as confirmed by ROSE. We usually complete EUS-FNAB after four needle passes in accordance with the report by Suzuki et al.14 However, when sufficient specimens were not confirmed by ROSE, we performed additional needle passes.
The following echoendoscopes and ultrasonography equipment were used: GF-UC240AL-5, GF-UCT260, EU-ME-1, and EU-ME-2 (Olympus Medical Systems, Tokyo, Japan). The needles were selected as follows. For patients with vessels near the puncture line, a conventional FNA needle was used because of its good penetration ability. Otherwise, the endoscopist randomly selected the needle. The Franseen Acquire 22-G needle (Boston Scientific, Marlborough, MA, USA) was used for EUS-FNB (Fig. 2). The cutting area of the Franseen needle was larger than that of a conventional needle. The needles used for EUS-FNA were an EchoTip 22-G (Cook Medical Inc., Winston Salem, NC, USA), EZ Shot 3 Plus 22-G (Olympus Medical Systems), and Expect 22-G (Boston Scientific).
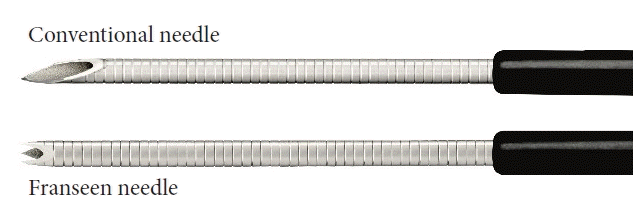
The Franseen needle used for endoscopic ultrasound-guided fine-needle biopsy. The cutting surface of the conventional fine-needle aspiration needle has a lancet shape. The area of the cutting surface becomes larger by adopting the Franseen design.
All procedures were performed by pancreaticobiliary specialists who had performed more than 200 EUS-FNAB procedures or by beginners under the guidance of pancreaticobiliary specialists.
Examination items
Patient characteristics (age, sex), UR-PC data (tumor size, location, and progression), and procedural outcomes (puncture route, puncture number, possibility of histological diagnosis, possibility of performing MSI evaluation, and adverse events) were compared between patients who underwent EUS-FNB and those who underwent EUS-FNA. Additionally, a multivariate analysis was performed to determine the factors that influenced the possibility of MSI evaluation. Tumor size was measured using computed tomography or EUS. Locally advanced UR-PC was determined under the following conditions: (1) invades/contacts the portal vein or superior mesenteric vein by >180° with a range of invasion/contact transcending the inferior duodenal angle; (2) invades/contacts the celiac artery or superior mesenteric artery by >180°; (3) invades/contacts the common hepatic artery with a range of invasion/contact extending to the celiac artery or proper hepatic artery; and (4) invades/contacts the aorta.15,16 In contrast, UR-PC was diagnosed by histology or cytology using EUS-FNAB. Cytology was classified on a scale of classes I to V (I, normal; II, atypical but benign; III, difficult to diagnose as benign or malignant; IV, suspected malignant; and V, malignant). Malignancy was defined as a class IV or V cytology.
Requirements for histological diagnosis and MSI evaluation
MSI evaluations for the three hospitals were performed by an external agency (Falco Holdings Co., Kyoto, Japan). If a histological specimen did not satisfy the conditions required for MSI evaluation, the specimen was not submitted to the outside agency for pathological judgment.
Histological diagnosis was made using all specimens submitted to the department of pathology. A formalin-fixed paraffin-embedded (FFPE) block was designated for each procedure performed at the Fukushima Medical University and Aizu Medical Center. Alternatively, an FFPE block was prepared for each puncture at the Ohtanishinouchi Hospital. To diagnose whether a tumor is malignant, the specimen must include tumor tissue that has not degenerated or crushed. Furthermore, when a specimen contains inflammatory contents, it becomes difficult to identify whether atypical cells are tumoral or reactive to inflammation.
The conditions for histological specimens required by the outside agency (Falco Holdings Co.) were as follows: more than 2,000 PC cells to extract DNA for MSI evaluation, PC cell content >50%, and more than five unstained PC slide specimens must be prepared. An MSI detection kit, called the Promega panel, was used to analyze MSI using five mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27). Although the Bethesda panel has been used for MSI evaluation for a long time,17 the Promega panel is superior to the Bethesda panel for MSI evaluation.18 In addition, unlike the Bethesda panel, the Promega panel does not require a normal tissue control.19-21 When more than two markers are MSI positive, MSI-high PC is diagnosed.
Statistical analyses
The Mann-Whitney U-test was used to compare continuous variables that did not conform to a normal distribution with ordinal variables. The Fisher exact test was used to compare nominal variables. Logistic regression was used to investigate the factors influencing the possibility of MSI evaluation. Statistical significance was set at p<0.05. EzR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)22 was used for all statistical analyses.
Ethical statements
This multicenter retrospective study was approved by the Institutional Review Board of Fukushima Medical University (IRB No: 2021-002). Patients were not required to provide informed consent because this study used anonymous clinical data obtained after each patient had agreed to medical activities by written consent. For full disclosure, the details of this study are published on the homepage of the Fukushima Medical University.
RESULTS
Patient characteristics, EUS-FNA, and EUS-FNB
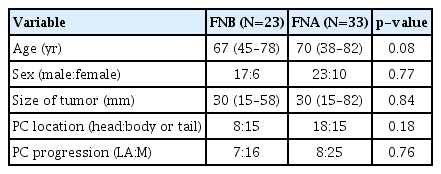
Patient characteristics, tumor size, PC location, and PC progression were not significantly different between patients who underwent FNB and those who underwent FNA (Table 1). Regarding the outcomes of EUS-FNAB, the puncture route, puncture number, and adverse events were not significantly different between the two groups (Table 2). Regarding FNA needles, Expect was used in 20 patients, EZ Shot 3 Plus was used in nine patients, and Echotip was used in four patients. Gastric bleeding at the puncture site was observed in one patient who underwent FNA. Bleeding was treated with endoscopic hemostasis using clips. The possibility of histological diagnosis was also not significantly different between the two groups (FNB 91.3% [21/23] vs. FNA 72.7% [24/33]; p=0.10). The possibility of MSI evaluation was significantly more frequent with FNB than with FNA (82.6% [19/23] vs. 45.5% [15/33], respectively; p<0.01). An MSI-high tumor was observed in only one patient who underwent FNA.
Comparison between EUS-FNB and FNA using Expect
Three needles were used in the patients who underwent EUS-FNA. However, Expect was used in most patients who underwent EUS-FNA. When patient characteristics and outcomes were compared between EUS-FNB and FNA using Expect, the results were similar to those of the comparison between EUS-FNB and FNA in all patients (Table 3). The possibility of histological diagnosis was not significantly different between FNB and FNA using Expect (91.3% [21/23] vs. 70.0% [14/20], respectively; p=0.12). The possibility of MSI evaluation using Expect was significantly higher in the FNB group than in the FNA group (82.6% [19/23] vs. 40.0% [8/20], respectively; p<0.01).
Factors influencing the possibility of MSI evaluation
In the univariate analysis, EUS-FNB was a significant factor influencing the possibility of MSI evaluation. The current guideline recommends three to four needle passes with FNA needles or two to three needle passes with FNB needles.23 Therefore, EUS-FNB and greater than three punctures were included in the multivariate analysis. As a result, EUS-FNB was the only significant factor influencing the possibility of MSI evaluation in the multivariate analyses (Table 4).
DISCUSSION
We investigated the efficacy of EUS-FNB with a Franseen needle for MSI evaluation. No significant difference in terms of sufficient tissue acquisition for histology was observed between patients who underwent EUS-FNB and those who underwent EUS-FNA. MSI evaluation can be performed more often with samples obtained using EUS-FNB than with those obtained using EUS-FNA.
MSI-high PC is rare. In previous reports with large sample sizes, MSI-high PC was detected in 0.5%–2% of patients.24-26 Consistent with previous reports, only one PC patient was diagnosed with MSI-high PC in this study. Although MSI-high PC is rare, pembrolizumab has shown dramatic results in some MSI-high PC patients.27,28 In a report by Obayashi et al.,28 a UR-PC patient with multiple lung metastases was treated with pembrolizumab, complete remission of the multiple lung metastatic lesions was confirmed, and the patient underwent surgical resection for PC. Therefore, sufficient tissue samples should be obtained for MSI evaluation in patients with UR-PC.
In this study, sufficient tissue acquisition for histology was achieved using EUS-FNA. However, in previous reports, more tissue was obtained with EUS-FNB than with EUS-FNA.10,11 In addition, other conditions were necessary, as described in the Methods section. Thus, EUS-FNB with a Franseen needle is appropriate to meet the conditions necessary for MSI evaluation. The difference in tissue sampling between a Franseen needle and a lancet-shaped conventional needle is thought to be caused by a difference in the cutting surface area; the Franseen needle has three cutting surfaces, whereas a conventional needle has only one. Therefore, the cutting area is larger for the Franseen needle than for the conventional needle.
This study has several limitations. First, it was an observational study with a small number of patients. However, according to the results of this study, the possibility of MSI evaluation was 82.6% and 45.5% in the FNB and FNA groups, respectively. A total of 52 patients were required to achieve an α error of 5% and a β value of 0.2. When the main outcome was the possibility of MSI evaluation, a minimum sample size was maintained. Second, the EUS-FNAB needles were not randomly assigned. Third, the handling of histological specimens differed across institutions. Because an FFPE block was made for each puncture at the Ohtanishinouchi Hospital, the number of tumor cells on the glass slide decreased, making it difficult to perform MSI evaluation. In the future, a multicenter prospective study should be conducted to verify the results of this study. Fourth, the volume of the UR-PC specimen was not numerically measured. Instead, the suitability of MSI evaluation was compared between patients who underwent EUS-FNA and those who underwent EUS-FNB using a Franseen needle. The histological diagnoses and possibility of MSI evaluation were evaluated by pathologists with considerable experience.
In conclusion, obtaining sufficient tissue samples for histological analysis is possible with EUS-FNA, similar to EUS-FNB. However, EUS-FNB using a Franseen needle is desirable for obtaining sufficient tissue samples for MSI evaluation and could aid in clinical decision-making regarding the treatment of patients with UR-PC.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We thank all the staff at the Department of Gastroenterology of Fukushima Medical University (Ohtanishinouchi Hospital and Aizu Medical Center), the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of the Fukushima Medical University Hospital.
Author Contributions
Conceptualization: TT, MS, HO; Data curation: TT, MS, HI, YT, YN; Formal analysis: TT, MS; Investigation: TT, MS, YH; Methodology: TT, MS; Project administration: TT, MS; Supervision: YH, GS, SM, TH, HO, RS, NK, HA, YS, HI, JN, MT, MH, TK, RK; Writing–original draft: TT, MS; Writing–review & editing: all authors.