AbstractBackground/AimsMucosal incision-assisted biopsy (MIAB) for tissue acquisition (TA) from subepithelial lesions (SELs) is emerging as an alternative to endoscopic ultrasound (EUS)-guided TA. Only a limited number of studies compared the diagnostic utility of MIAB and EUS for upper gastrointestinal (GI) SELs; therefore, we conducted this systematic review and meta-analysis.
MethodsA comprehensive literature search from January 2020 to January 2022 was performed to compare the diagnostic accuracy and safety of MIAB and EUS-guided TA for upper GI SELs.
ResultsSeven studies were included in this meta-analysis. The pooled technical success rate (risk ratio [RR], 0.96; 95% confidence interval [CI], 0.89ŌĆō1.04) and procedural time (mean difference=ŌĆō4.53 seconds; 95% CI, ŌĆō22.38 to 13.31] were comparable between both the groups. The overall chance of obtaining a positive diagnostic yield was lower with EUS than with MIAB for all lesions (RR, 0.83; 95% CI, 0.71ŌĆō0.98) but comparable when using a fine-needle biopsy needle (RR, 0.93; 95% CI, 0.83ŌĆō1.04). The positive diagnostic yield of MIAB was higher for lesions <20 mm (RR, 0.75; 95% CI, 0.63ŌĆō0.89). Six studies reported no adverse events.
INTRODUCTIONSubepithelial lesions (SELs) of the gastrointestinal (GI) tract arise from the muscularis mucosa, submucosa, or muscularis propria. SELs, although most commonly are incidental findings on endoscopy, can rarely present with bleeding, dysphagia, gastric outlet obstruction, and metastasis based on size, nature of the lesion, and location in the GI tract.1 The detection rate of SELs has increased recently owing to the increased use of screening endoscopies and the advent of technology.2 Although most SELs are benign, 15% can be malignant at presentation.3 Hence, appropriate identification and characterization of these lesions are of utmost importance.
Although SELs are routinely identified on endoscopy, endoscopic ultrasonography (EUS) is the first-line modality for characterizing SELs as it provides information regarding the layer of origin, intramural/extramural location, size and shape, echogenicity, vascularity, and associated lymphadenopathy. The initial mode of tissue acquisition (TA) for diagnosis was made using jumbo biopsy forceps with the bite-on-bite technique rather than the standard biopsy forceps. In a retrospective analysis, TA with jumbo biopsy forceps had a diagnostic yield of 60%, with a better yield compared to that of the EUS fine-needle aspiration (FNA) in lesions arising from the submucosal layer (65.1% vs. 37.5%) than the muscularis propria layer (40% vs. 57.1%), but with a higher risk of bleeding when a biopsy was performed on lesions arising from the fourth layer.4
With increasing availability, EUS-guided TA using FNA or fine-needle biopsy (FNB) is currently the most commonly employed method. However, the diagnostic yield of EUS-FNA is affected by the availability of rapid on-site evaluation by a cytopathologist and the size of the lesion, with lesions less than 2 cm having a poor diagnostic yield compared to that of the larger lesions.5,6 TA using EUS-FNB obviates the need for rapid on-site evaluation, requires fewer passes, and preserves tissue architecture. However, previous meta-analyses comparing TA using FNA and FNB needles have reported conflicting results.7-9
Since the original description of the technique by Yokohata et al.,10 mucosal incision-assisted biopsy (MIAB) or single incision with a needle knife has gained importance as an alternative method of TA. A mucosal incision line was chosen for this technique, and saline with 0.001% epinephrine was injected submucosally. A mucosal incision was made using an electrosurgical knife. After submucosal dissection, a biopsy of the exposed SEL was performed using conventional biopsy forceps, followed by closure of the mucosal incision with endoclips.11 In a meta-analysis, Dhaliwal et al.12 showed a high pooled diagnostic yield of MIAB and relatively shorter operating time. An MIAB variant, endoscopic submucosal dissection-assisted deep biopsy has shown a pooled diagnostic rate of 95% with a very low rate of adverse events (AEs).13 Given the high diagnostic yield of MIAB, it can serve as an alternative to EUS-guided TA for the diagnosis of upper GI SELs with minimal complications. Hence, the present systematic review and meta-analysis aimed to compare EUS-FNA/B and MIAB for the optimal method of TA.
METHODSInformation sources and search strategyThe Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Science Direct databases were searched from January 2000 to January 2022 for all relevant studies. The following keywords were used for the search: (EUS OR ŌĆ£Endoscopic ultrasoundŌĆØ) AND Subepithelial AND (MIAB OR Incision OR Biopsy OR ŌĆ£Needle knifeŌĆØ). Additionally, the reference lists of all identified trials, guidelines, and reviews on the topic were searched for relevant records. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and MetaŌĆÉAnalyses (PRISMA) guidelines.14
Study selectionTwo independent reviewers searched the titles and abstracts of the retrieved search records for the inclusion and exclusion criteria, followed by full-text screening of potential eligible citations. A third reviewer resolved disagreements. Studies included in this meta-analysis were comparative studies fulfilling the following PICO criteria: (1) Patients=upper GI SELs; (2) Intervention=use of MIAB or its variants, like submucosal tunneling for TA; (3) Comparison=EUS-guided TA using either FNA or FNB needle; and (4) Outcomes=procedural outcomes, diagnostic outcomes, and AEs. Only the original articles were included in the analysis. There was no bar on language, as long as the study outcomes were mentioned in the text. Non-comparative studies, conference abstracts, case series, and studies involving persons aged <18 years were excluded from the analysis.
Data extractionData extraction was independently performed by two investigators. A third reviewer resolved disagreements. Data were collected under the following headings: study author and year, number of patients, age distribution, type of intervention used and comparator arm, follow-up duration, outcomes, and AEs.
Definition of outcomesThe primary outcome of the analysis was a positive diagnostic yield, defined as the percentage of lesions in which a pathologist could make a confirmed diagnosis. The secondary outcomes included the technical success, procedural time, and AEs. Technical success was defined as access to the target tissue and obtaining of visible tissue specimens or fragments. AEs included the development of pain, bleeding, and perforation, which were directly related to the procedure. The procedural time was considered according to the definition of individual studies.
Statistical analysisDichotomous variables were analyzed using the risk ratio (RR) and Mantel-Haenszel test. A random-effects model was used irrespective of the presence of heterogeneity. The Q and I2 statistics were used to assess heterogeneity among the studies. A p-value of Q test <0.1 or the I2 value >50% was considered to be significant. Publication bias was assessed by visual inspection of funnel plots. A subgroup analysis was performed based on the size and location of the SEL. A sensitivity analysis was conducted using a leave-one-out meta-analysis, which excluded one study from each analysis to investigate each study's influence on the overall effect-size estimate and to identify influential studies. All statistical analyses were performed using the RevMan software (ver. 5.4.1; Cochrane Collaboration) and STATA software (ver. 17; StataCorp., College Station, TX, USA).
RESULTSStudy characteristics and qualityTotal 825 records were identified in the search, and 536 were screened after removing duplicates. Figure 1 shows the PRISMA flowchart of the article selection process. Seven studies17-23 were included in the meta-analysis. Table 1 summarizes the characteristics of the included studies. The majority of the studies were from Asia,17-21 one study was from Europe,22 and another multicenter study involved centers in North America and Europe.23 Three studies were prospective,18,20,21 three were RCTs1,19,23 and one was retrospective.21 The SEL was located in the stomach in most of the studies,17-19,21 while three studies20,22,23 included lesions in the esophagus and duodenum along with the stomach. The pooled mean age of the population was 61.1┬▒12.1 years. With respect to EUS-guided TA, four studies used FNA needles,17-19,22 two used FNB needles,20,23 and one used both.21 Among the RCTs, only one had a low risk of bias,23 whereas the other two had a moderate risk of bias (Fig. 2A).19,22 Among the non-randomized studies, one study had a low risk of bias,18 two had a moderate risk of bias,20,21 and one had a high risk of bias (Fig. 2B).17
Outcomes1) Technical successAll seven studies17-23 with 520 patients reported the technical success of both procedures. Both MIAB and EUS-guided TA had comparable technical success rates (RR, 0.94; 95% confidence interval [CI], 0.87ŌĆō1.03; I2=81%), with significant heterogeneity among the studies (Fig. 3). A sensitivity analysis using only RCTs19,22,23 also showed a comparable technical success between the two modalities (RR, 1.00; 95% CI, 0.94ŌĆō1.06; I2=0%).
2) Positive diagnostic yieldAll seven studies17-23 reported the outcome of diagnosis based on histology. The pooled rate of diagnostic yield with MIAB was 90.7% (95% CI, 84.7ŌĆō96.7; I2=71.8%), which was significantly higher than that of the EUS-guided TA 72.1% (95% CI, 60.7ŌĆō83.4; I2=78.5%). The pooled rate of diagnostic yields with EUS-FNA and EUS-FNB were 73.8% (95% CI, 58.4ŌĆō89.3; I2=85.1%) and 77.7% (95% CI, 59.8ŌĆō95.7; I2=78.7%), respectively. Use of EUS for TA from SELs was associated with a lower diagnostic yield compared to that of MIAB (RR, 0.83; 95% CI, 0.71ŌĆō0.98; I2=67%), with significant heterogeneity among the studies (Fig. 4). Meta-regression to assess the source of heterogeneity could not be performed because the number of studies included in the analysis was less than ten.
Heterogeneity can be due to differences in the needle used, size of the mass lesion, and location of the lesion. Hence, a subgroup analysis was conducted to compare the diagnostic rates of EUS-FNB and MIAB. Both methods were comparable in achieving a diagnosis without any heterogeneity (RR, 0.93; 95% CI, 0.83ŌĆō1.04; I2=0%) (Fig. 5A). Comparing both techniques with respect to the lesion size, MIAB was better than EUS-guided TA without any heterogeneity (RR, 0.75; 95% CI, 0.63ŌĆō0.89; I2=0%) (Fig. 5B). On sensitivity analysis of only RCTs,19,22,23 the diagnostic yield was comparable between the two modalities (RR, 0.91; 95% CI, 0.77ŌĆō1.07; I2=30%). Analyzing available data of only gastric SELs,17-21 the diagnostic yield was lower with EUS than with MIAB (RR, 0.77; 95% CI, 0.62ŌĆō0.97; I2=71%) (Fig. 5C). However, on subgroup analysis of three studies19-21 based on the location of the lesion in stomach, there was no difference in the diagnostic yield between lesions in upper stomach (RR, 0.89; 95% CI, 0.79ŌĆō1.01; I2=0%), middle stomach (RR, 0.88; 95% CI, 0.69ŌĆō1.11; I2=0%), and lower stomach (RR, 0.72; 95% CI, 0.49ŌĆō1.06; I2=15%).
3) Procedural timeOverall, five studies17-19,21,23 including 418 patients reported differences in procedural time with both techniques. There was no significant difference in the procedural time, although there was significant heterogeneity (mean difference [MD], ŌĆō4.53 seconds; 95% CI, ŌĆō22.38 to 13.31; I2=99%) (Fig. 6). A sensitivity analysis pooling data from only RCTs19,23 showed no difference in the procedural time (MD, ŌĆō5.81 seconds; 95% CI, ŌĆō14.53 to 2.91; I2=89%).
4) Procedural complicationsAmong the seven included studies,17-23 six did not report any AE associated with either procedure. In the study by Sanaei et al.,23 the rate of AEs was 7.69% (95% CI, 2.13ŌĆō24.14) in the EUS-FNB group as compared to 10% (95% CI, 3.45ŌĆō25.62) in the MIAB group (p>0.99). In the EUS-group, two patients had abdominal pain, whereas in the MIAB group, one had pain and two developed delayed bleeding within six days after the procedure. One of the bleeding episodes was self-limiting, whereas the other required arterial embolization.
5) Assessment of publication bias and leave-one-out analysisVisual assessment of the funnel plot (Supplementary Fig. 1) and EggerŌĆÖs test (Supplementary Table 1) showed the presence of publications for both technical success and diagnostic accuracy, but not for procedural time. A leave-one-out meta-analysis (Supplementary Fig. 2) showed a significant change in procedural time (Supplementary Fig. 2C). With the exclusion of the study by Jung et al.,17 EUS-guided TA was associated with a significantly shorter procedure duration than MIAB (MD, ŌĆō12.34 seconds; 95% CI, ŌĆō21.05 to ŌĆō3.64; I2=91%). Table 2 summarizes findings with confidence in the evidence.
DISCUSSIONThe present meta-analysis attests to the role of MIAB as an alternative to EUS-guided TA in upper GI SELs. The analysis showed a similar technical success rate with EUS-guided TA and MIAB (RR, 0.96; 95% CI, 0.89ŌĆō1.04), but a lower rate of diagnostic yield with EUS-guided TA (RR, 0.83; 95% CI, 0.71ŌĆō0.98). On subgroup analysis, EUS-FNB was comparable to MIAB with respect to the diagnostic yield (RR, 0.93; 95% CI, 0.83ŌĆō1.04). However, the diagnostic yield of EUS-guided TA was lower than that of MIAB for diagnosing lesions less than 20 mm (RR, 0.75; 95% CI, 0.63ŌĆō0.89). Both techniques were associated with significantly low AE rates. Although the procedural time was similar in both methods on overall analysis (MD, ŌĆō5.81 seconds; 95% CI, ŌĆō14.53 to 2.91), the study by Jung et al.17 was a significant outlier, and with its exclusion, the mean procedural time was lower with EUS (MD, ŌĆō12.34 seconds; 95% CI, ŌĆō21.05 to ŌĆō3.64).
The current guidelines by the European Society of Gastrointestinal Endoscopy (ESGE) recommend tissue diagnosis for all SELs with features suggestive of gastrointestinal stromal tumor (GIST), size >20 mm, associated high-risk stigmata on EUS, or prior to surgical resection.24 The European Society for Medical Oncology25 and the Japanese GIST Guideline Subcommittee26 recommend resection of GIST, even those <20 mm. Hence, tissue sampling for pathological and immunohistochemical analyses is a critical step in managing SELs. The ESGE recommends using either MIAB or EUS-guided TA for sampling SELs >20 mm in size. For SELs <20 mm in size, the ESGE recommends MIAB as the first choice, followed by EUS-guided TA.24 Dhaliwal et al.,12 in a meta-analysis on the outcome of MIAB for upper GI SELs, reported an overall pooled diagnostic yield of 89% without any heterogeneity. In the current meta-analysis, MIAB was associated with a higher chance of diagnosis than EUS-guided TA. Hence, the utility of MIAB in the diagnosis of SELs needs to be explored in larger studies.
Meta-analyses comparing TA with SELs using FNA and FNB needles have reported conflicting results. Two meta-analyses reported better diagnostic accuracy with FNB,7,8 while another meta-analysis reported no difference in accuracy with FNA/FNB or based on the choice of needle employed.9 The pooled rates of diagnostic yield in the present analysis with MIAB, EUS-FNB, and EUS-FNA were 90.7% (95% CI, 84.7ŌĆō96.7), 77.7% (95% CI, 59.8ŌĆō95.7), and 73.8% (95% CI, 58.4ŌĆō89.3), respectively. In subgroup analysis comparing the diagnostic rate of EUS-FNB with MIAB in the present study, both methods were comparable in achieving a diagnosis without any heterogeneity (RR, 0.93; 95% CI, 0.83ŌĆō1.04). Considering the overall quality of the tissue obtained, both EUS-FNB and MIAB may be superior to EUS-FNA for the histological diagnosis of SELs.
In a study analyzing the factors influencing the diagnostic yield of EUS-FNA for SELs, the diagnostic accuracy was 50% for lesions <20 mm and 91.6% for those >20 mm.6 Akahoshi et al.5 reported a diagnostic rate of 71% for lesions <20 mm, 86% for those between 20 mm and 40 mm, and 100% for those >40 mm. This is mainly because the smaller size of the lesion makes it difficult to target using EUS-guided TA techniques. In the present analysis, the diagnostic yield of EUS-guided TA was lower than that of MIAB for small SELs <20 mm (RR, 0.75; 95% CI, 0.63ŌĆō0.89). The low diagnostic yield of EUS-guided TA in the study by Kobara et al.18 may be attributed to the fact that approximately 74% of the SEL were smaller than 2 cm. Hence, for SELs <20 mm, MIAB may be considered the preferred option over EUS. However, this rule has a caveat. Kim27 proposed a classification method to determine whether GISTs were predominantly intramural or extramural. Those with predominant extramural components (types III and IV) may not be adequately sampled using MIAB, and EUS-guided TA may be the preferred modality in these situations.
The present analysis showed no significant difference in the procedural time (MD, ŌĆō4.53 seconds; 95% CI, ŌĆō22.38 to 13.31), although there was significant heterogeneity. Therefore, these findings should be interpreted with caution. In a leave-one-out meta-analysis, after excluding the influential study by Jung et al.,17 the procedural timing was significantly shorter with EUS. Thus, MIAB may be associated with longer procedure duration than EUS.
Dhaliwal et al.12 reported a pooled clinically significant post-procedural bleeding rate of 5.03% (95% CI, 0.4ŌĆō12.9, I2=57.43%) with MIAB, but no perforation. In the present analysis, six17-22 of the seven included studies reported no AEs with either of the procedures. Only the study by Sanaei et al.23 reported AEs, such as abdominal pain and bleeding associated with both techniques without any significant difference (p=1.0). This indicates the comparable safety of the two procedures. In terms of other available techniques for tissue diagnosis of SELs, Facciorusso et al.28 compared EUS-FNB with bite-on-bite biopsy using jumbo forceps. The sample adequacy and diagnostic accuracy were significantly higher with EUS-FNB (94.1% vs. 77.5% and 89.3% vs. 67.1%, respectively), with a lower bleeding rate (6.6% vs. 29.1%). However, there are no recent comparative studies that evaluated the outcome of MIAB with jumbo-forceps biopsy.
The findings of this study are important for several reasons. First, the overall diagnostic yield with MIAB is comparable to that of EUS-guided TA and is better for lesions <20 mm in size. MIAB can be performed during routine endoscopy and no advanced equipment is required. Second, lesion size does not affect the diagnostic yield of MIAB, while needle passage and aspiration of small-sized SELs may be challenging with EUS. Third, MIAB can be easily performed regardless of the anatomic location of the lesion in the stomach, provided a reasonable bulge is visualized endoscopically (type I and II according to KimŌĆÖs classification). In contrast, EUS-guided TA, especially FNA, can have a higher failure rate when the SEL is in the cardia or fundus because the stiff device has difficulty accessing these areas.17 To address the suboptimal diagnostic yield of EUS-FNA, a study was conducted using a forward-viewing echoendoscope, which showed a complete histological assessment in 93.4% of patients.29 However, a subsequent RCT30 reported comparable rates of histologic diagnosis between forward- and oblique-viewing echoendoscopes (80.5% vs. 73.2%; p=0.453).
There were a few limitations in the study, most inherent to any meta-analysis, which warrant further discussion. First, most of the studies were from a single center and two were retrospective. Second, the RCTs included in the analysis were underpowered to demonstrate a reasonable difference. Third, there was moderate to considerable heterogeneity in the studies with respect to the type of needle used and size of the mass lesions. Moreover, the definitions for procedural time varied among the studies, and one study did not define procedural time. Finally, economic considerations regarding the impact of sampling by MIAB or EUS-guided TA were beyond the scope of the current meta-analysis.
In conclusion, MIAB and EUS-guided TA have comparable technical success, diagnostic accuracy, and procedural time, but with significant heterogeneity. However, MIAB was better than EUS-guided TA for small lesions without heterogeneity. MIAB is an alternative to EUS-guided TA in clinical practice and may be the procedure of choice for SELs <20 mm in size. In centers where EUS expertise is unavailable, MIAB is an easy and safe alternative for EUS-guided TA. Large multicenter trials are the need of the hour to validate the findings of this meta-analysis.
Supplementary MaterialSupplementary Fig. 2. Leave-one-out meta-analysis for sensitivity analysis of all outcomes. Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.133.
Fig.┬Ā1.Preferred Reporting Items for Systematic Reviews and MetaŌĆÉAnalyses (PRISMA) flowchart for the study selection process. 
Fig.┬Ā2.Risk of bias assessment for randomized controlled trials (A) and non-randomized studies (B). 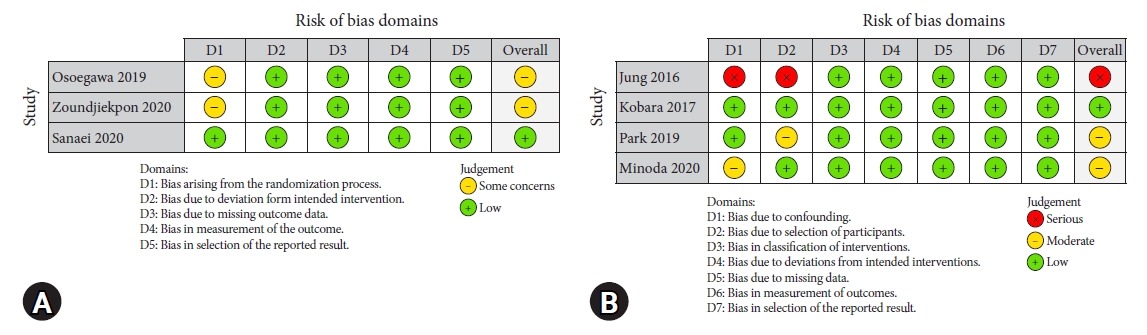
Fig.┬Ā3.Forest plot comparing the technical success between mucosal incision-assisted biopsy and EUS-guided tissue acquisition. EUS, endoscopic ultrasound; MIAB, mucosal incision-assisted biopsy; SD, standard deviation; IV, intravenous; CI, confidence interval. 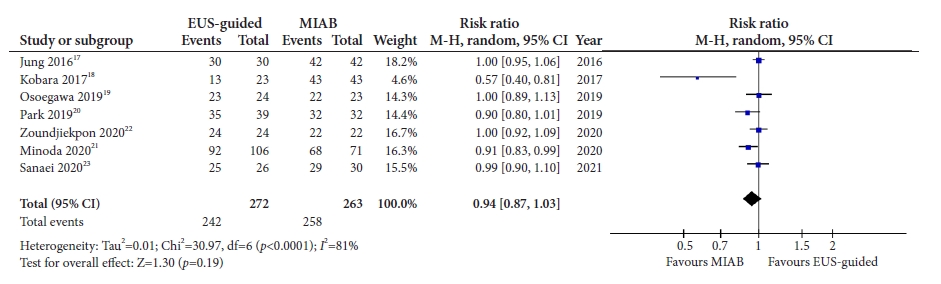
Fig.┬Ā4.Forest plot comparing the diagnostic yield between mucosal incision-assisted biopsy and EUS-guided tissue acquisition. EUS, endoscopic ultrasound; MIAB, mucosal incision-assisted biopsy; M-H, Mantel-Haenszel; CI, confidence interval. 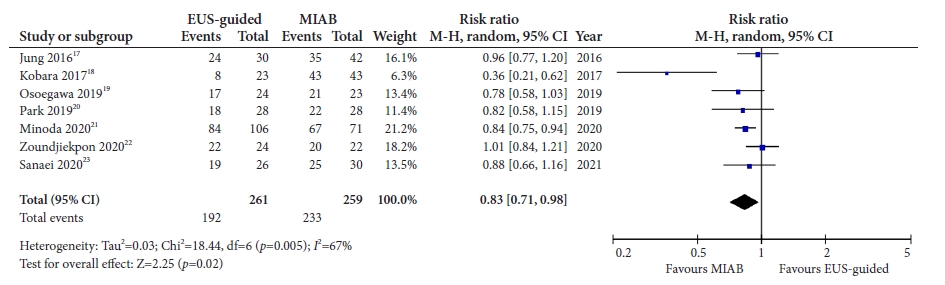
Fig.┬Ā5.Forest plot comparing the diagnostic yield between mucosal incision-assisted biopsy and EUS-guided tissue acquisition for subgroups. (A) Use of EUS-FNB. (B) Size of lesion <20 mm. (C) Gastric subepithelial lesions. EUS, endoscopic ultrasound; FNB, fine-needle biopsy; MIAB, mucosal incision-assisted biopsy; M-H, Mantel-Haenszel; CI, confidence interval. 
Fig.┬Ā6.Forest plot comparing the procedural time between mucosal incision-assisted biopsy and EUS-guided tissue acquisition. EUS, endoscopic ultrasound; MIAB, mucosal incision-assisted biopsy; M-H, Mantel-Haenszel; CI, confidence interval. 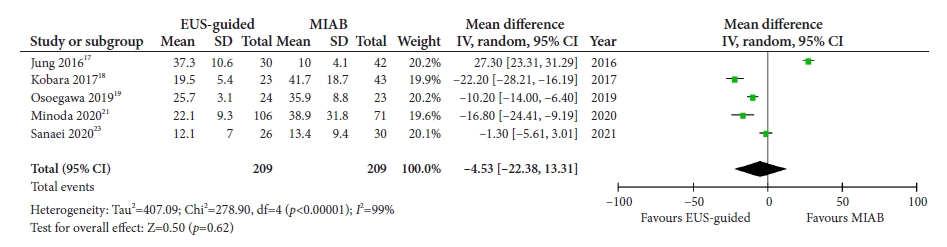
Table┬Ā1.Study characteristics of included studies
Values are presented as mean┬▒standard deviation or median (interquartile range). M/F, male/female; S/E/D, stomach/esophagus/duodenum; MIAB, mucosal incision-assisted biopsy; EUS, endoscopic ultrasound; FNA, fine-needle aspiration; G/L/H/O, gastrointestinal stromal tumor/leiomyoma/heterotopic pancreas/others; FNB, fine-needle biopsy; RCT, randomized controlled trial. Table┬Ā2.Summary of findings and grade of evidence PICO criteria: Population, patients with upper gastrointestinal subepithelial lesions; Intervention, use of mucosal incision-assisted biopsy or its variants for tissue acquisition; Comparison, EUS-guided tissue acquisition; a)Outcomes. CI, confidence interval; MIAB, mucosal incision-assisted biopsy; EUS, endoscopic ultrasound; RR, risk ratio; MD, mean difference. REFERENCES1. Humphris JL, Jones DB. Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol 2008;23:556ŌĆō566.
2. Hawes R, Fockens P, Varadarajulu S. Endosonography. 4th ed. Philadelphia: Elsevier; 2018.
3. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005;37:635ŌĆō645.
4. Buscaglia JM, Nagula S, Jayaraman V, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc 2012;75:1147ŌĆō1152.
5. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol 2007;13:2077ŌĆō2082.
6. Attila T, Ayd─▒n ├¢. Lesion size determines diagnostic yield of EUS-FNA with onsite cytopathologic evaluation for upper gastrointestinal subepithelial lesions. Turk J Gastroenterol 2018;29:436ŌĆō441.
7. van Riet PA, Erler NS, Bruno MJ, et al. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: a systemic review and meta-analysis. Endoscopy 2021;53:411ŌĆō423.
8. Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: a meta-analysis. Gastrointest Endosc 2020;91:14ŌĆō22.
9. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc 2016;30:2431ŌĆō2441.
10. Yokohata N, Tamegai Y, Tokuhara M, et al. 3 case of gastric SMT (submucal tumor) which was diagnoced before operation: open biopsy with ESD (endoscopic submucal dissection) for interstind SMT. Prog Dig Endosc 2007;70:82ŌĆō83.
11. Ihara E, Matsuzaka H, Honda K, et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc 2013;5:191ŌĆō196.
12. Dhaliwal A, Kolli S, Dhindsa BS, et al. Clinical efficacy and safety of mucosal incision-assisted biopsy for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol 2020;33:155ŌĆō161.
13. Dhaliwal A, Kolli S, Dhindsa BS, et al. Diagnostic yield of deep biopsy via endoscopic submucosal dissection for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol 2020;33:30ŌĆō37.
14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
15. Higgins JP, Altman DG, G├Ėtzsche PC, et al. The Cochrane CollaborationŌĆÖs tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
16. Sterne JA, Hern├Īn MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919.
17. Jung YS, Lee H, Kim K, et al. Using forceps biopsy after small submucosal dissection in the diagnosis of gastric subepithelial tumors. J Korean Med Sci 2016;31:1768ŌĆō1774.
18. Kobara H, Mori H, Nishimoto N, et al. Comparison of submucosal tunneling biopsy versus EUS-guided FNA for gastric subepithelial lesions: a prospective study with crossover design. Endosc Int Open 2017;5:E695ŌĆōE705.
19. Osoegawa T, Minoda Y, Ihara E, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: a randomized cross-over study. Dig Endosc 2019;31:413ŌĆō421.
20. Park J, Park JC, Jo JH, et al. Prospective comparative study of endoscopic ultrasonography-guided fine-needle biopsy and unroofing biopsy. Dig Liver Dis 2019;51:831ŌĆō836.
21. Minoda Y, Chinen T, Osoegawa T, et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: a propensity score matching analysis. BMC Gastroenterol 2020;20:19.
22. Zoundjiekpon V, Falt P, Fojtik P, et al. Endosonography-guided fine-needle aspiration versus ŌĆ£Key-Hole BiopsyŌĆØ in the diagnostics of upper gastrointestinal subepithelial tumors: a prospective randomized interventional study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2020;164:63ŌĆō70.
23. Sanaei O, Fern├Īndez-Esparrach G, De La Serna-Higuera C, et al. EUS-guided 22-gauge fine needle biopsy versus single-incision with needle knife for the diagnosis of upper gastrointestinal subepithelial lesions: a randomized controlled trial. Endosc Int Open 2020;8:E266ŌĆōE273.
24. Deprez PH, Moons LM, O╩╝Toole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022;54:412ŌĆō429.
25. Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20ŌĆō33.
26. Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416ŌĆō430.
27. Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: advantages and hurdles. World J Gastrointest Endosc 2015;7:192ŌĆō205.
28. Facciorusso A, Crin├▓ SF, Ramai D, et al. Comparison between endoscopic ultrasound-guided fine-needle biopsy and bite-on-bite jumbo biopsy for sampling of subepithelial lesions. Dig Liver Dis 2022;54:676ŌĆō683.
|
|
