See commentary "Role of endoscopic ultrasound in the secondary prevention of gastric varices" in Volume 56 on page 50 AbstractBackground/AimsGastric varices (GV) are present in 25% of cirrhotic patients with high rates of rebleeding and mortality. Data on endoscopic ultrasound (EUS)-guided treatment in severe liver disease (model for end stage liver disease sodium [MELD-Na] >18 and Child-Turcotte-Pugh [CTP] C with GV) are scarce. Thus, we performed a retrospective comparison of endoscopic glue injection with EUS-guided therapy in cirrhotic patients with large GV.
MethodsA retrospective study was performed in the tertiary hospitals of India. A total of 80 patients were recruited. The inclusion criteria were gastroesophageal varices type 2, isolated gastric varices type 1, bleeding within 6 weeks, size of GV >10 mm, and a MELD-Na >18. Treatment outcomes and complications of endoscopic glue injection and EUS-guided GV therapy were compared.
ResultsIn this study, the patientsŌĆÖ age, sex, liver disease severity (CTP, MELD-Na) and clinical parameters were comparable. The median number of procedures, injected glue volume, complications, and GV obturation were better in the EUS group, respectively. On subgroup analysis of the EUS method (e.g., direct gastric fundus vs. paragastric collateral [PGC] coil placement), PGC coil placement showed decreased coil requirement, less injected glue volume, decreased luminal coil extrusion, and increased successful GV obturation.
INTRODUCTIONGastric varices (GV) are present in 15% to 25% of patients with liver cirrhosis, with gastroesophageal varices type 1 (GOV 1) being the most common type.1 Although the incidence of GV bleeding is low (10%ŌĆō20%), that of GV rebleeding is very high, with an associated mortality of up to 30%.1,2 GV can also be present in patients without intra-hepatic portal hypertension, as seen in left sided portal hypertension, which include pancreatitis (acute or chronic), pancreatic malignancy, myeloproliferateive disorders (polycythemia vera, essential thrombocytosis), or in certain hereditary disorders.1-3 As portal pressure determines the outcomes of bleeding and development of complications (decompensation: ascites, hepatic encephalopathy [HE]) in patients with cirrhosis, bleeding in esophageal varices (EV) is relatively proportional to portal pressure. Therefore, treatment for EV is focused on lowering portal pressure. However, in patients with GV bleeding, portal pressure may be normal or even lower (<10ŌĆō12 mmHg), which is why targeting the portal pressure is insufficient in the treatment of GV bleeding. Moreover, the presence of a thick mucosal cover makes it difficult to control the bleeding in some cases. As such, procedures which decrease portal pressure (e.g., transjugular intrahepatic portosystemic shunt [TIPS]) without any combination are unsatisfactory in GV. Given all this, the standard treatment of GV has been focused on the localized cessation of the blood flow in the collateral or shunt (using balloon occlusion with retrograde transvenous obliteration [BRTO] or plug-assisted retrograde transvenous obliteration) (Supplementary Fig. 1).4-11
Risk factors for bleeding from the GV include the following5: (1) stigmata of recent hemorrhage (ulcer, visible bleeding, or red spot); (2) advanced or severe liver disease: decompensated liver disease, high model for end stage liver disease sodium (MELD-Na), or acute on chronic liver failure (ACLF); (3) location of the varices: isolated gastric varices type 1 (IGV1) > GOV2 >GOV1; (4) presence of portal vein thrombosis; and (5) presence of portal hypertensive gastropathy.7-13
As there is scarcity of data on the ideal treatment modality in cases of GV with severe liver disease (MELD-Na >18, Child-Turcotte-Pugh [CTP]-C, and ACLF), TIPS and BRTO are relatively contraindicated. Fortunately, endoscopic ultrasound (EUS)-guided angiotherapy and endoscopic glue injection (EGI) can be indicated treatment modalities in such patients. To that end, we performed a study comparing EGI (standard treatment) with EUS-guided glue and coil injection in severe liver disease patients (MELD-Na >18 and CTP-C) with large GV.
METHODSA retrospective analysis of prospectively collected data was done after obtaining an ethical approval and the valid consent of all patients diagnosed with GV. The included patients had previously received EGI as the first line treatment and were planned for the management of large GV as a secondary endotherapy (after the initial episode of GV bleeding). Data were collected from June 2016 to December 2019, and the follow-up period lasted for 12 months post-procedure to observe the possible development of delayed complications, including rebleeding, development of new GV, disappearance of GV, and increasing size of EV. There was no cross over in between the groups, if the patient developed rebleeding. Such patients were managed with the same endoscopic treatment or were rescued with a BRTO procedure. Furthermore, since the primary outcome of the study was rebleeding, it was initially decided to make the study groups more homogenous.
All patients were on beta blockers, and dose titration was done as per the standard of care. In case of acute bleeding, patients were started on injectable vasopressors (terlipressin or octreotide infusion) and antibiotics, as per the standard, and blood products were transfused if required.10-13
GV were classified as per Sarin classification1,5 on the location and size as follows: (1) small, size <5 mm; (2) moderate, 5ŌĆō10 mm; and (3) large, >10 mm.
Inclusion criteria(1) GV (GOV2 and IGV1), (2) recent bleeding from the GV, (3) GV size >10 mm, (4) severe liver disease defined as ACLF (as per APASL classification) or advanced liver disease (MELD-Na >18, CTP-C), and (5) being unfit for TIPS due to previous episodes of HE or having a high MELD score (MELD-Na >18).
Exclusion criteria(1) Unwilling patients; (2) pregnancy; (3) small GOV1; (4) advanced hepatocellular carcinoma defined as Barcelona clinic liver cancer (BCLC) staging system stage C or D; (5) advanced splenoportal venous thrombosis (portal vein and splenic vein, portal vein and superior mesenteric vein); (6) portosystemic shunting, especially hepatopulmonary syndrome or intracardiac shunt that increases the probability of systemic embolization, which was diagnosed on the basis of clinical signs/symptoms, imaging, or on echocardiography; (7) previous history of glue embolization during EGI; and (8) large EV (>5 mm) with signs of high risk (red color signs, cherry red spots, platelet plug), especially in the lower esophagus in cases where transesophageal puncture is being planned.
The selection criteria for patients in the EUS group were as follows: (1) being referred from the treating gastroenterologist for EUS-guided therapy, (2) recurrence of GV bleeding after EGI session, and (3) persistence of large GV after EGI session (assessed endoscopically as size >10 mm and soft consistency on probing).
A subgroup analysis was also performed for those who underwent EUS-guided gastric varices therapy (GVT), wherein one group was targeted in the fundus and the other group in the paragastric collateral (PGC). Patients who were selected for PGC were chosen on the basis of their portovenous anatomy (either with computed tomography [CT] venography or magnetic resonance venography), including those with a large PGC and/or large perigastric shunt (short gastric vein and posterior gastric vein), in addition to the gastric submucosal component. If no shunt or collateral was noted, then the gastric fundus was targeted. Both groups were compared, and the results were analyzed.
Procedure details are further provided in Supplementary Material 1.
Follow-up endoscopyA follow-up endoscopy and EUS Doppler were done at the end of 3 months of treatment, or earlier in case of rebleeding. Variceal treatment was defined as complete if there were no remnant GV or hardening of GV on endoscopic examination, or if no color flows were noted on Doppler examination. Meanwhile, treatment was defined as incomplete in case of the persistence soft varices on endoscopic examination or if persistence of blood flow was noted on Doppler. In such cases, a repeat injection was done, wherein repeat coil and glue was injected for cases that underwent EUS initially.
Study outcomesThe primary outcome was rebleeding after the initial treatment. The secondary outcome measure was complications, such as embolization, death, or sepsis.
Rebleeding was defined as presence of hematemesis and/or melena, with findings of a hemoglobin (Hb) drop of >2 g/dL from the baseline, stigmata of recent GV hemorrhage, and no other etiology for the cause of bleeding. Embolization was defined as the documentation of an embolus on post-procedural imaging, in addition to presence of symptoms. Death was determined to be related to the procedure if no other etiology could be found. Sepsis was defined on the basis of the presence of symptoms (fever, tachycardia), blood parameters (leukocytosis, increased serum procalcitonin), and positive blood cultures.
Statistical analysisPatient and GV characteristics, procedure details, and procedural outcomes were summarized as frequencies for categorical variables, and proportions, means with standard deviations, and medians with interquartile ranges for continuous variables. Categorical variables were then compared between the EGI and EUS-GVT groups using either FisherŌĆÖs exact test or the Chi-square test as indicated, whereas continuous variables were compared using the Wilcoxon rank sum test. For all analyses, statistical significance was set at p<0.05, and data were compiled using Microsoft Excel and analyzed using IBM SPSS ver. 22.0 software (IBM Corp., Armonk, NY, USA).
Post-procedural complicationsPatients were assessed for the development of post-procedural complications, including perforation, bleeding, pain, infection, or systemic embolism. These were monitored at the following time periods: 6 hours post-procedure until discharge, one week after the procedure, monthly for two months, and then 6 months post-procedure. Perforation was defined as the presence of free air under the diaphragm. Bleeding was defined as Hb drop of >2 g/dL post-procedure that required blood transfusion. Systemic embolism was defined as the presence of symptoms with a documented embolus on imaging (CT scan of thorax).
RESULTSAmong the 321 patients presenting with GV bleeding, a total of 80 patients were included for analysis after exclusion (Fig. 1). Data was then retrospectively collected and compared during the study period. Patient characteristics are presented in Table 1. The etiology of liver disease in both groups included alcohol-related liver disease, non-alcoholic steatohepatitis (NASH), hepatitis B, hepatitis C, and others (autoimmune-related and cryptogenic etiologies), which were comparable between the two groups, showing no statistical differences. Endoscopic treatment was performed as a secondary management (after the first bleeding episode) in both groups. The median follow-up period was 193 days and 201 days, respectively, which was comparable between two groups, showing no statistical differences.
However, there was a significant decrease in the number of endoscopy sessions (4 vs. 1.2), volume of glue injected (6 mL vs. 2 mL), and rebleeding (5 vs. 0), respectively, in both procedures. Moreover, all of these factors were comparatively less in the EUS-GVT group (Table 2).
Complications were noted in both groups, including 12 patients in the EGI group (five moderate, seven mild) and five in EUS-GVT group (all mild). Four patients complained of fever (two in EGI group and two in EUS-GVT group), and five patients complained of post-procedural abdominal pain (two in EGI group and three in EUS-GVT group). Five patients in the EGI group had rebleeding, which was managed with repeat EGI; however, three patients required further BRTO. Conversely, no rebleeding was noted in the EUS-GVT group. Regarding mortalities, three patients in the EGI group and two patients in the EUS-GVT group died due to causes unrelated to the procedure on follow-up (one due to pneumonia, one due to spontaneous bacterial peritonitis, one due to acute kidney injury, two due to HE, and none of them died due to bleeding or embolization). All these patients died after 3 months of the procedure but were still included in the final analysis.
In the subgroup analysis of the EUS-GVT group, it was noted that the volume of glue (2.5 mL vs. 1.5 mL), number of coils (28 vs. 24), coil extrusion in the gastric lumen (3 vs. 0), and incomplete obliteration of the GV (2 vs. 0) were noted less in the PGC subgroup than in the gastric fundus group, respectively. In contrast, more time was taken to complete the procedure (40 min vs. 55 min) in the PGC group (Table 3).
DISCUSSIONGV are present in 25% of patients with cirrhosis and portal hypertension. In acutely bleeding patients, pharmacotherapy with somatostatin, terlipressin, and octreotide could be empirically administered, but the efficacy of these drugs is doubtful. During the follow-up of these patients, the addition of beta blockers to the standard endoscopic therapy has also shown to be beneficial. In contrast, standard endoscopic therapies used for EV, including sclerotherapy and band ligation, are less effective for GV and are associated with high complication rates. In fact, prospective randomized control studies comparing EGI with ligation techniques and sclerotherapy have shown that EGI therapy was superior to both of them. However, although EGI has been determined as the standard of care for GV, it has the following limitations when compared to EUS-GVT: (1) obliteration of GV (63% vs. 84%), (2) recurrence of GV (18% vs. 9%), (3) late rebleeding (16% vs. 12%), and (4) complications (50% vs. 25%), respectively.13-18
EUS has been reported to diagnose GV and portal collateral vessels in portal hypertension, perigastric veins, PGC, and portal hypertensive gastropathy. Color Doppler EUS can also display if the main feeding vein system (inflow vein) receives blood from the left gastric vein trunk, posterior gastric vein, or short gastric veins. Moreover, it helps in the assessment of the outflowing venous drainage via the gastrorenal shunts (Figs. 2, 3). In cases with extensive PGCs, EUS can help in directing treatment when targeting the GV in the fundus or combining it with injections in the PGC (Figs. 3, 4).17,18
In a large retrospective study of 152 patients, simultaneous use of EUS-guided glue and coil for GV showed an increased success rate of therapy and a decreased rebleeding rate of approximately 3%, as compared to other studies using conventional treatments with an endoscopic glue, which have reported a rebleeding rate of 15% to 25%.19 The study also witnessed lower rates of complications in glue or coil embolizations and mortalities. The studyŌĆÖs high success rate and minimal complications can be attributed to the high expertise of the endoscopists and a high-volume center.19
In a previous case series by Romero-Castro et al.18 regarding the management of GV, the perforating veins were targeted, and the study concluded that targeting the gastric perforating veins or collaterals was feasible. Furthermore, the new method described was not associated with increased complications, as compared to conventional EGI. In our study, we found that targeting the PGCs was feasible and was associated with a decreased glue volume and coil number requirements. Another international study also reported that EUS-guided injection of glue into a GV led to the formation of a subendothelial string in the outflowing vein, which was covered by endothelial and fibroblastic cells.20 Therefore, we believe that EUS-guided glue injection into the PGC is an option in patients with especially prominent PGCs. For such cases, targeting the draining vessel (inflowing veins) can produce the blockage of inflow into the GV, while using a lesser amount of glue and fewer coils, leading to obturation (Figs. 3, 4, and Supplementary Video 1).18,19,21-23 Despite this, there are limitations to the use of EUS for the management of GV. First, technical expertise is required for these procedures, since vascular endotherapy is technically challenging and has been associated with complications if performed amateurly. Second, there is also a problem in the technique in cases of a transgastric approach for management, since the deployment of coils and injection of the glue may become challenging when there is an acute angulation at the tip of scope, thereby making the deployment of the glue and coils difficult. Lastly, there is also the concern regarding which technique should be used for endotherapy (transgastric vs. transesophageal). Since current data have yet to clarify which route is better and should be preferred, further trials are required to confirm this. For example, in cases of co-existing large EV, the transesophageal route is not preferred due to risk of rupture. Whereas in our study, we compared both routes, finding that the transesophageal route was slightly better. Moreover, the PGC is difficult to identify especially due to presence of a large number of collaterals around the splenic hilum and gastric fundus area. In addition, there is an increased chance of complications, including failure to identify the correct vein and wrong injection into splenic veins that adjoin the spleen and the surrounding structures.20,22-27
In conclusion, EUS-guided placement of the coil and glue is superior in efficacy, requires less number of treatment sessions, and is associated with less complications, as compared to EGI treatment of GV in patients with severe liver disease. Moreover, a lesser number of endoscopy sessions and rebleeding episodes also decreases the risk for GV bleeding in critically ill patients (high MELD-Na and CTP score). Although paragastric collateral targeting seems superior and safe, further studies are needed to validate these findings.
Supplementary MaterialSupplementary Fig. 1. Algoithmic approach in the management of gastric varices. Supplementary Video 1. EUS-guided gastric varices therapy (EUS-GVT) (https://doi.org/10.5946/ce.2021.119.v001). Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2021.119.
Fig.┬Ā1.Flow chart of the included patients. GV, gastric varices; PVT, portal vein thrombosis; HCC, hepatocelluar carcinoma; SVT, superior mesenteric vein thrombosis; BRTO, balloon occlusion with retrograde transvenous obliteration; MELD-Na, model for end stage liver disease sodium; TIPS, trans┬Łjugular intrahepatic portosystemic shunt; EUS, endoscopic ultrasound. 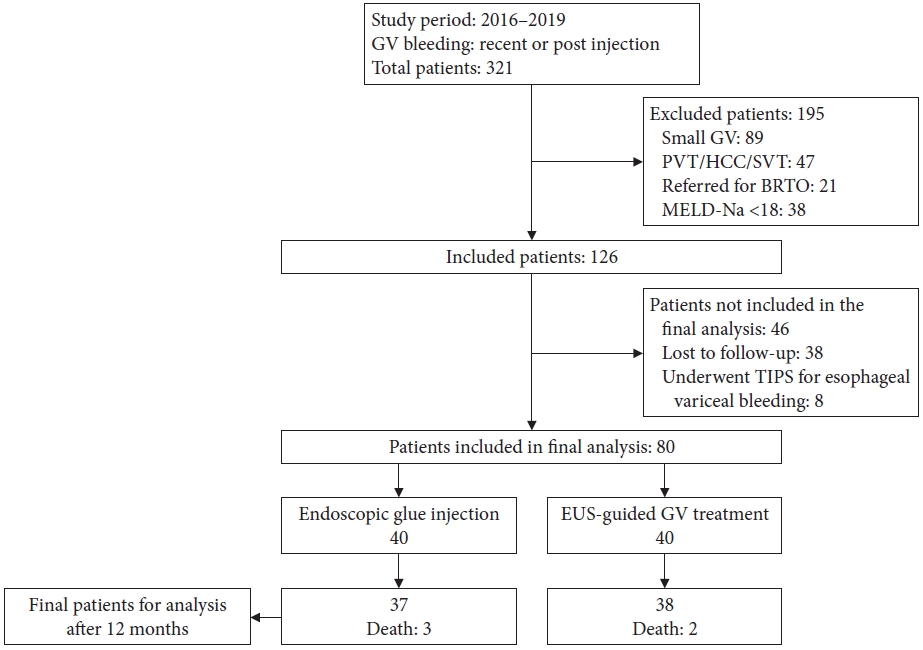
Fig.┬Ā2.Endosonographic images for the mucosal component of the gastric varices and connecting collateral (A), the large perisplenic collateral (B), an embolization coil deployed into the gastric varices (C), and after the coil has been deployed inside the gastric varices (D). 
Fig.┬Ā3.The image shows the combination of a cartoon (A, D) showing various components (mucosal, shunt, and paragastric collateral) of the gastric varices (GV). (B) The coronal computed tomography scan section shows that the gastrorenal shunt (GRS) drains the GV. The red arrow points to the submucosal portion (paragastric collateral), and the white arrow points to the mucosal portion of the GV. (C) Endoscopic appearance of the same GV. (E) EUS appearance of the same GV, with the mucosal GV and collateral. LR, lienorenal; PEP, phrenic esophageal pericardiac vein; PGC, paragastric collateral; PV, portal vein; SGV, short gastric vein; SV, splenic vein. 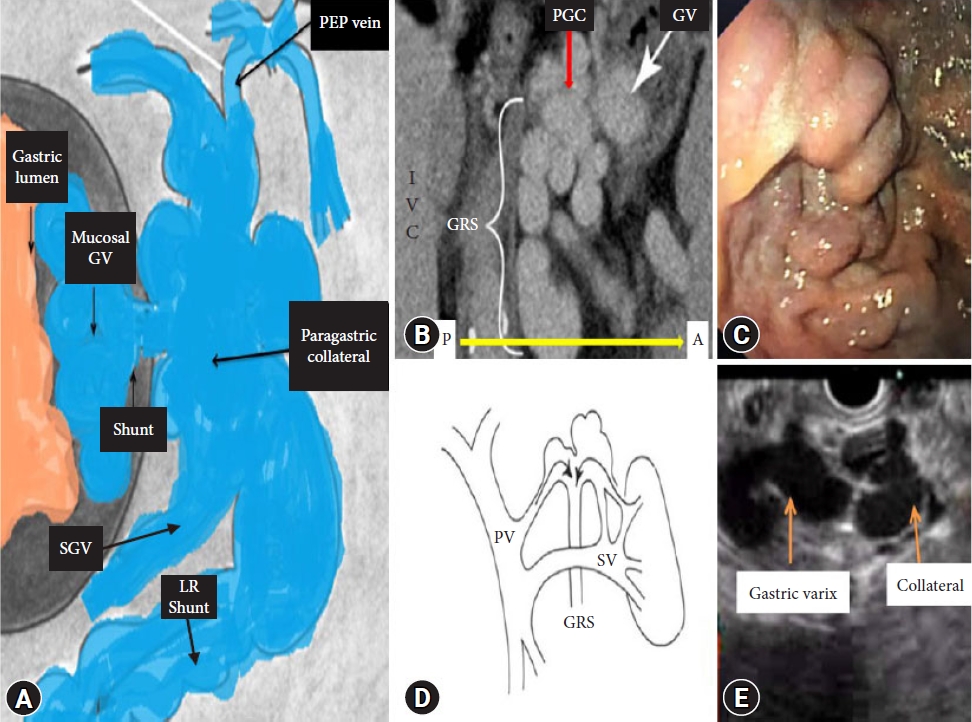
Fig.┬Ā4.Images showing isolated gastric varices type 1 (IGV1). (A) Endoscopic appearance of a large IGV1 with stigmata of recent hemorrhage. (B) The coronal computed tomography scan section shows the portovenous phase and the draining veins of IGV1. (C) A coil is deployed in the gastric fundus of the IGV1, and the collateral is seen distant to the coil. (D) A non-contrast coronal computed tomography scan shows the coil in the same IGV1. PV, portal vein; SV, splenic vein. 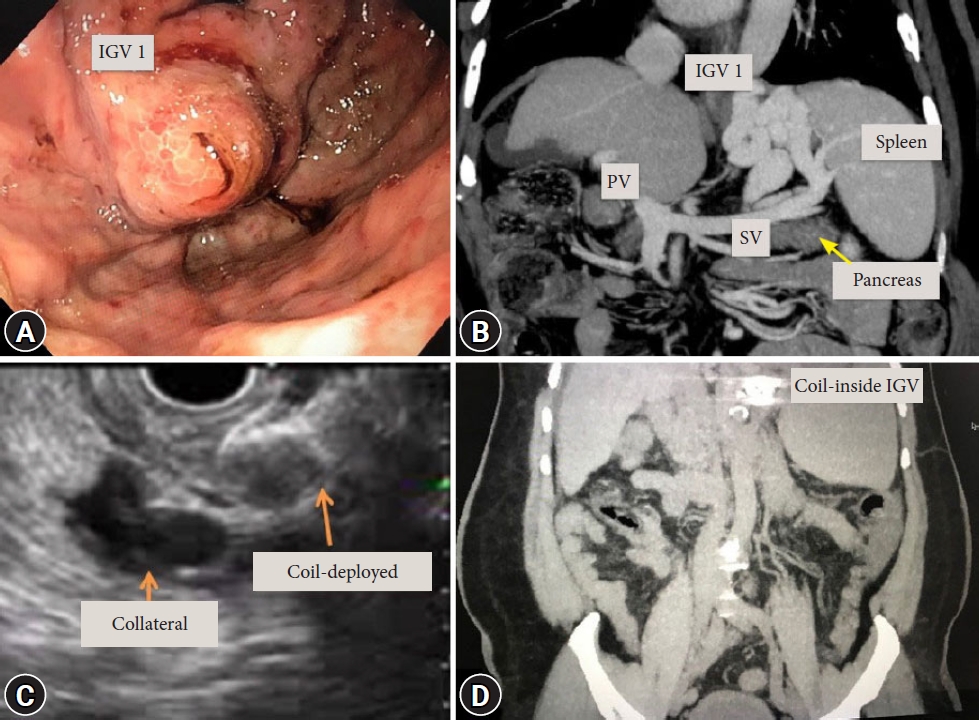
Table┬Ā1.Characteristics of endoscopic management of gastric varices: EGI vs EUS-GVT Table┬Ā2.Comparison between endoscopic modalities: EGI and EUS-GVT Table┬Ā3.Treatment outcomes according to the endoscopic ultrasound routes REFERENCES1. Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343ŌĆō1349.
2. Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology 1997;25:307ŌĆō312.
3. Pereira P, Peixoto A. Left-sided portal hypertension: a clinical challenge. GE Port J Gastroenterol 2015;22:231ŌĆō233.
4. Jamwal K, Padhan RK, Sharma A. Refractory variceal bleeding: approach to management (mini review). World J Gastroenterol Hepatol Endosc 2020;3:1ŌĆō3.
5. Lahoti S, Catalano MF, Alcocer E, et al. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc 2000;51:331ŌĆō333.
6. Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc 1986;32:264ŌĆō268.
9. Weilert F, Binmoeller KF. EUS-guided vascular access and therapy. Gastrointest Endosc Clin N Am 2012;22:303ŌĆō314.
10. Saraswat VA, Verma A. Gluing gastric varices in 2012: lessons learnt over 25 years. J Clin Exp Hepatol 2012;2:55ŌĆō69.
11. Watanabe K, Kimura K, Matsutani S, et al. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology 1988;95:434ŌĆō440.
12. Mishra SR, Sharma BC, Kumar A, et al. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol 2011;54:1161ŌĆō1167.
13. Weilert F, Binmoeller KF. New endoscopic technologies and procedural advances for endoscopic hemostasis. Clin Gastroenterol Hepatol 2016;14:1234ŌĆō1244.
14. McCarty TR, Bazarbashi AN, Hathorn KE, et al. Combination therapy versus monotherapy for EUS-guided management of gastric varices: a systematic review and meta-analysis. Endosc Ultrasound 2020;9:6ŌĆō15.
15. Robles-Medranda C, Oleas R, Valero M, et al. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices versus coiling alone: a randomized trial. Endoscopy 2020;52:268ŌĆō275.
16. L├┤bo MR de A, Chaves DM, DE Moura DTH, et al. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol 2019;56:99ŌĆō105.
17. Mohan BP, Chandan S, Khan SR, et al. Efficacy and safety of endoscopic ultrasound-guided therapy versus direct endoscopic glue injection therapy for gastric varices: systematic review and meta-analysis. Endoscopy 2020;52:259ŌĆō267.
18. Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc 2013;78:711ŌĆō721.
19. Bhat YM, Weilert F, Fredrick RT, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc 2016;83:1164ŌĆō1172.
20. Iwase H, Suga S, Morise K, et al. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc 1995;41:150ŌĆō154.
21. Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc 2007;66:402ŌĆō407.
22. Kakutani H, Hino S, Ikeda K, et al. Use of the curved linear-array echo endoscope to identify gastrorenal shunts in patients with gastric fundal varices. Endoscopy 2004;36:710ŌĆō714.
23. Bick BL, Al-Haddad M, Liangpunsakul S, et al. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg Endosc 2019;33:1837ŌĆō1845.
24. Jamwal K, Sharma M, Sarin S, et al. A comparitive analysis of endoscopic management of gastric varices: is EUS guided treatment safe, more efficacious and cost effective as compared to standard treatment in advanced liver disease? J Gastroenterol Hepatol 2019;34(Suppl 3):72.
25. Jamwal K, Sharma A, Padhan R, et al. EUS in management of gastric varices: targeting paraesophageal collateral is safe and better in severe liver disease as compared to gastric varices itself. Gastroenterology 2020;158(Suppl 1):SŌĆō1478.
|
|
|||||||||||||||||||||||||||||||||||||||||||||
