See commentary "Waterjet-assisted endoscopic submucosal dissection in the colorectum: safe and effective?" in Volume 55 on page 755 AbstractBackground/AimsColorectal endoscopic submucosal dissection (ESD) is burdened by its associated high risk of adverse events and long procedure time. Recently, a waterjet-assisted knife was introduced to simplify and speed up the procedure. The aim of this study was to evaluate the efficacy and safety of waterjet-assisted ESD (WESD) compared to that of the conventional ESD (CESD) technique.
MethodsThe charts of 254 consecutive patients who underwent colorectal ESD between January 2014 and February 2021 for colorectal neoplasms were analyzed. The primary outcome was the en-bloc resection rate. Secondary outcomes were complete and curative resection rates, the need to switch to a hybrid ESD, procedure speed, the adverse event rates, and the recurrence rates.
ResultsApproximately 174 neoplasias were considered, of which, 123 were removed by WESD and 51 by CESD. The en-bloc resection rate was higher in the WESD group (94.3% vs. 84.3%). Complete resection rates and curative resection rates were similar. The need to switch to a hybrid ESD was greater during CESD (39.2% vs. 13.8%). Procedure speed and adverse event rates were similar. During follow-up, one recurrence occurred after a WESD.
INTRODUCTIONEndoscopic submucosal dissection (ESD) is an operative endoscopic procedure that allows en-bloc resection of early colon and rectal neoplasias, including large ones. Furthermore, ESD has a lower recurrence rate than that of endoscopic mucosal resection (EMR).1 However, in Western countries, ESD is not used ubiquitously due to its steep learning curve,2 high risk of adverse events, and increased time required.3 The two crucial points determining technical feasibility are the favorable position of the neoplasia with an easier approach and a clear distinction of the submucosal and muscular layer.
Regarding the last point, the maintenance of tissue traction and good submucosal exposure during dissection is an important factor for an effective, safe, and rapid dissection. To improve submucosal exposure and speed up the procedure, several traction techniques have been developed.4 In particular, Bordillon et al.5 have recently demonstrated that the double-clip traction technique allows enthusiastic results regarding procedure speed and oncological outcomes.
A few years ago, a waterjet-assisted knife was introduced with the aim of simplifying and speeding up the procedure. This technology allows the injection of fluids with pressures above 20 bars (other than injection based on needles or roller pumps) through a centric axial nozzle in the electrode tip of the HybridKnife (HK) (Erbe Elektromedizin GmbH, Tuebingen, Germany). The result is that with the same device, it is possible to cut, dissect, and inject fluids into the submucosa, as well as coagulate the vessels beforehand, or in case of bleeding, there is no need to change accessories during the procedure.6,7 Thanks to these features, the HK has proved capable of reducing procedure time compared to that of the standard technique, in the removal of mucosal and submucosal lesions of the stomach, with the same efficacy and safety.8,9
The aim of the current retrospective study was to evaluate the efficacy and safety of waterjet-assisted ESD (WESD) in comparison with that of the conventional ESD (CESD) technique in patients with colorectal neoplasia. The study was performed in one tertiary referral center in Italy with a volume of ESD procedures of approximately 100 cases annually.
METHODSPatientsThis study is a retrospective analysis of a prospectively collected database. All consecutive patients who underwent ESDs for non-recurrent colorectal neoplasias at Arcispedale Santa Maria Nuova (Reggio Emilia, Italy) between January 2014 and February 2021 were enrolled.
Lesions with a high risk of significant fibrosis were excluded, as well as post-EMR or postsurgical recurrence, dysplastic lesions of inflammatory bowel disease, and submucosal lesions. Periprocedural data for all patients were collected. Pre-procedural data included age, sex, American Society of Anesthesiologists physical status classification, and the diameter and area of the colorectal neoplasia. The morphology was registered based on the Paris-Classification. Lesions that were diagnosed as Paris IIa were referred to as laterally spreading tumors (LST), and subspecified as granular type and nonŌĆÉgranular type.10,11 Procedural data were included, such as the switch to hybrid ESD, procedure time, intraprocedural perforation, and severity of submucosal fibrosis. A hybrid ESD was defined as a snare resection following circumferential incision and partial submucosal dissection, which simplifies the procedure when the submucosal layer is unclear or not easily approachable.12 Submucosal fibrosis was classified as F0 (no fibrosis), F1 (mild fibrosis: appearing as a white web-like structure in the blue submucosal layer), and F2 (severe fibrosis: appearing as an absence of submucosal layer and white structure between mucosal and muscular layers).13 Post-procedural data included histology of the endoscopically resected specimen, en-bloc resection rate, complete resection rate, delayed perforation and bleeding, post-polypectomy syndrome, need for surgery, length of hospital stay, and recurrence rate on follow-up.
OutcomesThe primary outcome of the study was the en-bloc resection rate, defined as resection of the neoplasia in a single piece. Secondary outcomes included the rate of complete and curative resection, the need to switch to hybrid ESD, procedure speed, incidence of adverse events, recurrence rate, and length of hospital stay. Complete resection was defined as tumor resection in a single piece with negative lateral and vertical margins. A curative resection was achieved when both the lateral and vertical margins of the specimen were free of neoplasia, and when there was no submucosal invasion deeper than 1,000 microns, lymphatic invasion, vascular involvement, or poorly differentiated components. Procedure speed (mm┬▓/min) was calculated by dividing the surface area of the resected specimen (mm┬▓) by procedure time (min). Procedure time was calculated starting from the first submucosal injection until complete detachment of the neoplasia. Resected specimen surface area was calculated using the ellipse formula: area (mm┬▓)=longest axis (mm) by shortest axis (mm)├Ś0.25├ŚŽĆ.
Adverse events included intraprocedural perforation, delayed perforation, delayed bleeding, post-polypectomy syndrome, and fever with bacteremia. Intraprocedural perforation was defined as the section of the colorectal muscular layer with direct visualization of peritoneal fat or retroperitoneal space. Delayed perforation was defined as the presence of free air in the peritoneal or retroperitoneal space detected by abdominal radiography or abdominopelvic computed tomography after colorectal ESD achievement. Delayed bleeding was defined as overt hematochezia or melena arising from the resection site over 6 hours after completion of the colorectal ESD. Post-polypectomy syndrome is defined as the development of abdominal pain, fever, leukocytosis, and peritoneal inflammation in the absence of perforation. Fever with bacteremia was defined as a body temperature greater than 37.5 degrees in conjunction with evidence of bacteria in blood samples. Recurrence was defined as the presence of neoplastic tissue in the site of ESD diagnosed during follow-up colonoscopies.
Statistical analysisCategorical variables are expressed as proportions, and were analyzed using the Fisher exact test or chi-square test (Žć┬▓). Continuous variables are expressed as means with standard deviations, and were analyzed using the Student t-test. A p<0.05 was considered statistically significant. Univariate and multivariate logistic regression analyses were performed to assess the factors of en-bloc resection. Odds ratios and 95% confidence intervals were calculated to evaluate the predictors of en-bloc resection. All data analyses were performed using MedCalc (ver. 19.8; MedCalc Software Ltd., Ostend, Belgium).
ProceduresAll procedures were performed by two endoscopists (P.C. and F.A.) who had performed about 20 colorectal and 50 gastric ESDs at the time of their first enrolled case.
Patients fasted for at least 12 hours before ESD and were monitored with pulse oximetry during the procedure. Continuous electrocardiography monitoring was also performed on patients with known cardiac disease or arrhythmias. All patients underwent deep sedation with fentanyl and midazolam, and in selected cases, with propofol administered by an anesthesiologist. All procedures were performed using a single-channel Fujifilm zoom gastroscope or slim zoom video colonoscope (EG-760Z or EC-760ZP; Fujifilm, Tokyo, Japan) with an attached transparent tip hood (ST hood, DH-29CR or Top hood, SHM; Fujifilm). Several types of electrosurgical knives were used for the dissection: a waterjet system-assisted knife (HybridKnife T-type; Erbe Elektromedizin GmbH) (Fig. 1) and three types of electrosurgical knives without a waterjet system (DualKnife, Triangle Tip Knife, KD10Q; Olympus, Tokyo, Japan) connected to an electrosurgical unit (VIO 3 and VIO 200D; Erbe Elektromedizin GmbH) with carbon dioxide insufflation. In hybrid ESD, a single use 15 mm or 25 mm diameter electrosurgical snare was used (Snare Master; Olympus) to complete the resection.
For placing the incision, ENDO CUT Q mode effect 2 and ENDO CUT I mode effect 2 were used in highly and poorly vascularized lesions, respectively. For submucosal dissection, ENDO CUT Q mode effect 2 or SWIFT COAG mode effect 4 were used in poorly and moderately vascularized lesions, while SPRAY COAG mode effect 5 was used in highly vascularized lesions.
Before starting the procedure, a Boston Bowel Preparation Scale14 of 9 was required to ensure good visualization of the lesion and to reduce the risk of infectious adverse events. Saline solution with small amounts of indigo carmine and epinephrine was used to lift the submucosa off the muscle layer. Hemostatic forceps (Hot Biopsy; Olympus) to treat and prevent bleeding were used only in the case of large vessels, with FORCED COAG mode effect 3 and SOFT COAG mode effect 6.
ESD techniqueIn Azienda USL-IRCCS di Reggio Emilia, colorectal ESDs are performed using the conventional or tunnel technique.15,16
The detailed steps of CESD were as follows: (1) the lesion was positioned at 5 oŌĆÖclock; (2) a circumferential incision around the lesion was created; (3) progressive dissection of the lesion following the first incision and working forward or in retroversion was performed; and (4) complete removal of the lesion was ensured.
The detailed steps of the tunnel technique were as follows: (1) the lesion was positioned at 5 oŌĆÖclock; (2) a first incision on the proximal side of the lesion was placed; (3) a second incision on the distal side of the lesion was placed; (4) dissection of the lesion from the distal incision to the proximal incision was performed, creating a tunnel; (5) the dissection was widened within the tunnel toward the lateral margins; and (6) lateral margins were cut, resulting in removal of the lesion.
The choice to perform a WESD or a CESD was at the discretion of the operator, and related to the availability of devices. In difficult situations, such as a very unstable, uncomfortable, or inaccessible position, when severe submucosal fibrosis was encountered, or when clinical instability of the patient was observed due to perforation or difficult sedation, hybrid ESD or underwater ESD was used as a rescue technique. The hybrid ESD consisted removal of the lesion with a snare after performing a circumferential incision followed by a partial submucosal dissection, thus reducing the execution time; its usefulness in difficult situations has been demonstrated.12 Underwater ESD consisted performing the dissection after having filled the colonic segment with water to obtain more stability, good exposure, and hydration of the submucosal layer despite being affected by fibrosis.17
After resection, an adequate assessment of the defect was performed to identify the presence of any prominent vessels or muscular layer injuries. The prominent vessels were coagulated with diathermic forceps, while muscular injuries were closed with clips.
Ethical statementsAll patients provided written informed consent to undergo ESD. This retrospective study was first approved by Clinical Trials and Statistics Unit, SC Infrastructure, Research and Statistics, Azienda USL-IRCCS Di Reggio Emilia (No. 2021/0005027), then by the Area Vasta Emilia Nord Ethics Committee on 14 January 2021, and was registered on ClinicalTrials.gov on 3 March 2021 (NCT04780256).
RESULTSCharacteristics of the enrolled patientsBetween January 2014 and February 2021, 261 colorectal lesions were removed by ESD in 254 patients at Arcispedale Santa Maria Hospital (Reggio Emilia, Italy). In total, 75 recurrent neoplasias were noted after endoscopic resection, while three dysplastic lesions of inflammatory bowel diseases and nine submucosal tumors were excluded. Eventually 174 neoplasias in 167 patients were considered (mean age, 69.5 years; 56.9% males). Of these, 123 lesions in 119 patients were removed by WESD and 51 lesions in 50 patients by CESD. In two cases, where two lesions were found, the two different techniques were used (Fig. 2).
Baseline characteristics of the patients and lesions are shown in Table 1. With regard to the localization of the lesions, the neoplasias removed with HK were more frequently localized in the rectum (52.0%), while those removed with CESD were more frequently localized in the right colon (45.1%) (p=0.032). In contrast, the morphology of the lesions was moderately balanced in the two treatment groups, although granular LSTs were removed mainly by WESD (50.4%), while non-granular LSTs were removed by CESD (49.0%). The surface area of the lesions removed with HK was significantly larger than those removed with CESD (37.7 mm vs. 28.3 mm, p=0.001). The submucosal fibrosis rate was comparable in the two groups, as well as the histopathological results, and in particular pT1 diagnoses (28.5% treated by WESD and 23.5% by CESD).
Study outcomesOutcomes of the two groups are summarized in Table 2. Univariate and multivariate logistic regression analyses are summarized in Table 3.
En-bloc resection rate was higher in the WESD group than that of the CESD group (94.3% vs. 84.3%, p=0.032). Complete resection and curative resection rates were similar in the two groups (WESD 87.0% vs. CESD 80.4%, p=0.266 and WESD 73.2% vs. CESD 68.6%, p=0.544, respectively).
The need to switch to a hybrid ESD was greater during ESDs performed without the HK (39.2% vs. 13.8%, p<0.001). In univariate logistic regression analyses, WESD (p=0.039) and absence of submucosal fibrosis (p=0.043) were significantly associated with en-bloc resection. Multivariate logistic regression analyses showed that only WESD (odds ratio, 3.27; 95% confidence interval, 1.01ŌĆō10.69; p=0.049) was an independent factor related to en-bloc resection.
Procedure speed was evaluated only in ESD procedures and not in those converted to hybrid ESD. Similar values were observed with the two techniques (WESD 13.4 mm2/min vs. CESD 14.7 mm2/min, p=0.492). With regard to adverse events, a low overall incidence was observed (7.5%) with no differences between the two groups. Six perforations (three per group) were observed, which were all managed endoscopically. Thirty-nine patients were referred for surgery, because of non-curative pT1 resection; none was a consequence of an adverse event.
Follow-up data was available for 83 patients treated with WESD and 28 treated with CESD, with a mean value of 16.6 and 13.7 months, respectively. No patient had recurrence in the CESD group while only one patient had a local recurrence after WESD. The procedure for the patient with local recurrence took very long because of persistent bleeding and difficult sedation; the neoplasia was removed with a hybrid ESD in a piecemeal fashion. The recurrence was subsequently successfully treated by WESD, and no further recurrence was noted. Length of hospital stay was significantly greater in the WESD group than that of the CESD group (2 days vs. 1.3 days, p<0.001).
DISCUSSIONESD is an endoscopic resection technique for the removal of early neoplasia of the gastrointestinal tract; recently, this technique is being increasingly performed across the world.1 ESD provides a higher rate of en-bloc and curative resection compared to EMR. Despite this, it remains a technically difficult and time-consuming procedure, especially when applied to the colorectal endoluminal tract.2,3 Position stability, good submucosal visualization, and adequate knife angle are key factors to make the procedure quicker, easier, and safer.
Many techniques have been proposed to improve these aspects; in particular, the use of traction techniques or specific devices.4,5 Furthermore, in particularly complex situations, such as presence of severe submucosal fibrosis or clinical instability of the patient, rescue techniques have been proposed, such as the hybrid technique. The hybrid technique consists of resection of the lesion with a snare after a subtotal submucosal dissection, which inevitably leads to a reduction in procedural time and also a reduction in en-bloc resection rate.12
Furthermore, to facilitate ESD, the HK has been introduced in the past years. This device is a modified knife equipped with an integrated waterjet system that allows injection of saline into the submucosa at any time, using high-pressure injection of low fluid volumes and electrosurgical cutting and coagulation at the same time. This technique was first evaluated in an animal model. These studies showed that the use of the new waterjet-assisted knife accelerates ESD and increases its safety and efficacy.6,7 Subsequently, a randomized study on humans comparing conventional and WESD in early gastric cancer management showed that ESD with or without HK were comparably effective and safe techniques, but that ESD with HK was faster, simpler, and required fewer instrument changes, thus decreasing procedure time.8 As far as we know, the efficacy and safety of HK in the lower digestive tract has been recently evaluated in humans but never compared with that of CESD.18
In this study we retrospectively compared the procedural outcomes obtained from colorectal ESDs performed with HK (WESD) and without HK (CESD). The principal outcome measured was the en-bloc resection rate. The overall result was 91.4%, which represents a result in line with those published in Asian studies. This result underlines the trend toward a comparable technical expertise between Asian and Western endoscopists.19,20 In this study, the use of HK allowed the attainment of an even higher en-bloc resection rate (94.3% vs. 84.3%, p=0.032). This finding could be biased by the higher prevalence of right colon lesions removed with CESD. Localization to the right colon is a risk factor for en-bloc resection failure.21 Contrastingly, HK allows performance of rapid sequences of injection and cutting, which guarantee greater control on the cutting of the margins that is essential to obtain an R0 resection and consequently an en-bloc resection (Fig. 3). Complete and curative resection rate, 85.1% and 71.8%, respectively, did not differ between the two groups of treatment. These results were found to be in line with recently published non-Asian series,5 which unequivocally show an improvement compared to the past.
In a total sample of 175 cases, a switch to hybrid ESD was necessary in 37 cases. Hybrid ESD was used as rescue technique for several reasons, especially severe submucosal fibrosis, but also difficult positions and clinical instability due to perforation or difficult sedation. The switch to hybrid ESD was less necessary when HK was used (13.8% vs. 39.2%). The higher incidence of perforation and severe fibrosis encountered during CESD, even if not statistically significant, could in part explain this result. Moreover, the peculiarities of the HK could also play an important role. In fact, the possibility of performing a frequent submucosal injection without changing the device allows good visualization of the submucosa during the whole procedure, thus reducing the risks of muscular injury and perforation even when severe fibrosis is present (Fig. 4). Furthermore, the centric position of the nozzle allows for a precise injection without the need for repositioning the electrode for subsequent cutting. This fits into the workflow of alternating injection and dissection.
Regarding procedural speed, a similar value was observed when using or not using HK. Conversely, Zhou et al.8 demonstrated that HK allows for a quicker ESD of early gastric cancers. In our sample, dissection speed was probably markedly conditioned by the use of hemostatic forceps during procedure. The rate of use of hemostatic forceps in the WESD group was significantly higher than that in the CESD group (32.5% vs. 15.7%, p=0.023). Our explanation for this finding is that WESD has been performed predominantly for rectal lesions and large lesions (Table 1), which are characterized by marked vascularization that requires frequent prophylactic hemostasis as well as treatment of bleeding vessels. Indeed, analyses stratified for location and size show that the need of hemostatic forceps was significantly higher in the rectum than in any other location (39.5% vs. 17.2%, p<0.001), and in lesions >40 mm in size (44.8% vs. 16.8%, p<0.0001).
In this study, a reduced speed of the procedure compared to Japanese and recent European studies was observed.5,22 Although, in our opinion, en-bloc resection has a greater impact on procedural costs. En-bloc resection reduces the need for subsequent operative colonoscopies and surgical interventions for incomplete or non-curative resections, with consequent reduction in costs for the health system; above all, it allows patient healing with low risks and without the need for repeated interventions.
Regarding safety, results showed a low rate of adverse events (6.9%) and no difference between the two groups of treatment. Overall perforation rate was 3.4%, and no patients were referred to surgery for management of an adverse event. On the contrary, surgery was necessary for 37 patients because of non-curative pT1 resection.
During a mean follow-up of 15.6 months, one recurrence occurred after a difficult ESD performed with HK for a highly vascularized lesion. The recurrent lesion was successful treated by WESD. Length of hospital stay was longer in patients who underwent WESD, probably because WESD was used to remove larger neoplasias. Patients with colonic neoplasias larger than 3 cm scheduled for ESD were usually hospitalized for at least one day.
This study has several limitations. First, the study was retrospective in nature. Second, the sample size was small. Third, the decision to use HK or not was based on operator preference, without any established criteria; the different electrode sizes and geometries resulted in different current densities, resulting in different extents of dissective or coagulative properties of the instrument. An electrode of the shape of the DualKnife or Triangle tip knife results in higher current density than that of the thicker electrode of the HK, when using comparable voltages and modulation. This has an impact on the comparability between the two groups. However, as far as we know, this is the first study comparing the safety and efficacy between WESD and CESD for colorectal neoplasia to date. However, the most obvious potential study flaw is the historical bias. We tested the bias by comparing the outcome of first 20 ESD enrolled with the last 20 ESD. The comparison did not show any significant differences, and we could exclude any learning curve effect on the final outcome (switch to hybrid ESD of first 20 cases vs. last 20 cases: 10% vs. 20%, respectively, p=0.661).
In conclusion, in this retrospective evaluation of colorectal ESD performed in an Italian center, we can state that ESD is efficient and safe, with outcomes comparable to those of Asian studies. The use of HK in colorectal ESDs allows for a high rate of en-bloc resections, and less frequently requires a rescue switch to hybrid ESD compared to that of CESD. Finally, this device seems to facilitate ESD, and is probably useful for beginners and in challenging situations.
Fig.┬Ā2.Study flowchart. ESD, endoscopic submucosal dissection; WESD, waterjet-assisted ESD; CESD, conventional ESD. 
Fig.┬Ā3.(AŌĆōC) Progressive sequences of injection and cutting during the distal incision of a rectal lesion. 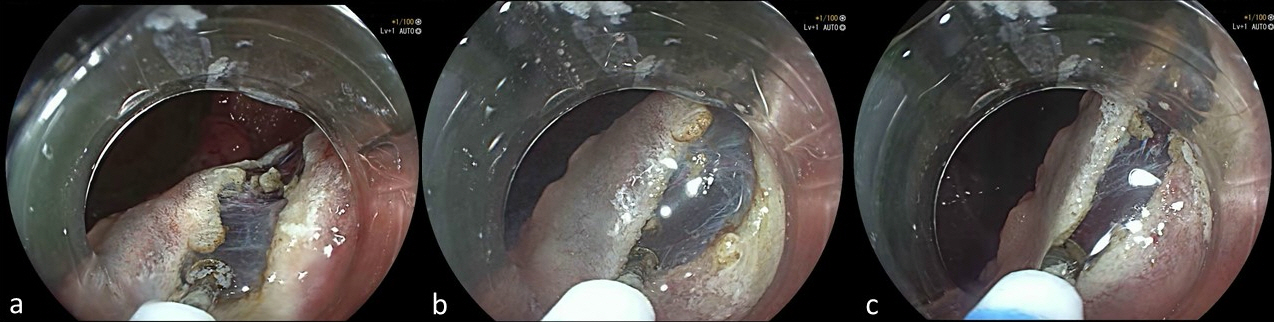
Fig.┬Ā4.Progressive sequences of submucosal injection improving the visualization of the submucosal layer in mild (F1) (AŌĆōC) and severe (F2) (DŌĆōF) submucosal fibrosis. 
Table┬Ā1.Baseline patientŌĆÖs and lesionŌĆÖs characteristics Values are presented as mean┬▒standard deviation or number (%). ESD, endoscopic submucosal dissection; WESD, waterjet-assisted ESD; CESD, conventional ESD; ASA, American Society of Anesthesiologists; LST-G, laterally spreading tumors granular type; LST-NG, laterally spreading tumors nonŌĆÉgranular type. Table┬Ā2.Therapeutic outcomes of ESD Table┬Ā3.Univariate and multivariate logistic regression analysis of factors associated with en-bloc resection
REFERENCES1. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015;47:829ŌĆō854.
2. Kato M, Gromski M, Jung Y, et al. The learning curve for endoscopic submucosal dissection in an established experimental setting. Surg Endosc 2013;27:154ŌĆō161.
3. Lee EJ, Lee JB, Lee SH, et al. Endoscopic submucosal dissection for colorectal tumors: 1,000 colorectal ESD cases: one specialized institute's experiences. Surg Endosc 2013;27:31ŌĆō39.
4. Abe S, Wu SYS, Ego M, et al. Efficacy of current traction techniques for endoscopic submucosal dissection. Gut Liver 2020;14:673ŌĆō684.
5. Bordillon P, Pioche M, Wallenhorst T, et al. Double-clip traction for colonic endoscopic submucosal dissection: a multicenter study of 599 consecutive cases (with video). Gastrointest Endosc 2021;94:333ŌĆō343.
6. Neuhaus H, Wirths K, Schenk M, et al. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc 2009;70:112ŌĆō120.
7. Yahagi N, Neuhaus H, Schumacher B, et al. Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy 2009;41:340ŌĆō345.
8. Zhou PH, Schumacher B, Yao LQ, et al. Conventional vs. waterjet-assisted endoscopic submucosal dissection in early gastric cancer: a randomized controlled trial. Endoscopy 2014;46:836ŌĆō843.
9. Zhou JQ, Tang XW, Ren YT, et al. Endoscopic submucosal tunnel dissection of upper gastrointestinal submucosal tumors: a comparative study of hook knife vs hybrid knife. World J Gastroenterol 2017;23:1843ŌĆō1850.
10. Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570ŌĆō578.
11. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3ŌĆō43.
12. Okamoto K, Muguruma N, Kagemoto K, et al. Efficacy of hybrid endoscopic submucosal dissection (ESD) as a rescue treatment in difficult colorectal ESD cases. Dig Endosc 2017;29 Suppl 2:45ŌĆō52.
13. Matsumoto A, Tanaka S, Oba S, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol 2010;45:1329ŌĆō1337.
14. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc 2010;72:686ŌĆō692.
15. Zou J, Chai N, Linghu E, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for rectal laterally spreading tumors. Surg Endosc 2021;35:4356ŌĆō4362.
16. Hayashi Y, Miura Y, Yamamoto H. Pocket-creation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumors. Dig Endosc 2015;27:534ŌĆō535.
17. Yoshii S, Akasaka T, Hayashi Y, et al. "Underwater" endoscopic submucosal dissection: a novel method for resection in saline with a bipolar needle knife for colorectal epithelial neoplasia. Surg Endosc 2018;32:5031ŌĆō5036.
18. Draganov PV, Aihara H, Karasik MS, et al. Endoscopic submucosal dissection in North America: a large prospective multicenter study. Gastroenterology 2021;160:2317ŌĆō2327.
19. Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74ŌĆō86.
20. Daoud DC, Suter N, Durand M, et al. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: a systematic review and meta-analysis. World J Gastroenterol 2018;24:2518ŌĆō2536.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
