Efficacy and safety of intragastric balloon for obesity in Korea
Article information
Abstract
Background/Aims
Intragastric balloon (IGB) is the only available endoscopic bariatric and metabolic therapy in Korea. End-ball (Endalis) has the longest history of clinical use among the IGBs available in Korea. However, little clinical data on this system have been reported. In this study, we aimed to evaluate the efficacy and safety of End-ball in Korea.
Methods
We performed a retrospective cohort study of patients who underwent IGB insertion (End-ball) from 2013 to 2019. Demographic and anthropometric data were collected. The efficacy and safety of IGB treatment were analyzed.
Results
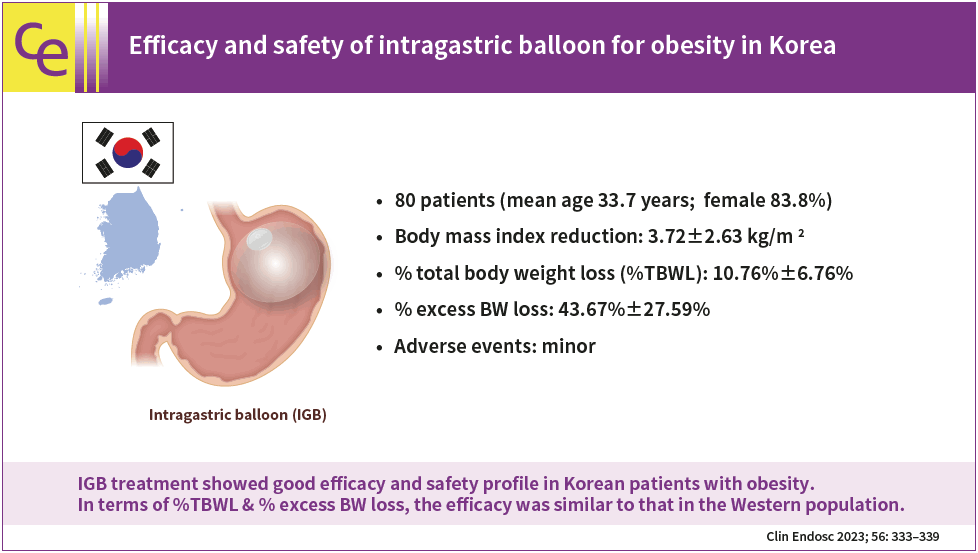
In total, 80 patients were included. Mean age was 33.7 years and 83.8% were female. Initial body mass index was 34.48±4.69 kg/m2. Body mass index reduction was 3.72±2.63 kg/m2 at the time of IGB removal. Percent of total body weight loss (%TBWL) was 10.76%±6.76%. Percentage excess body weight loss was 43.67%±27.59%. Most adverse events were minor, and 71.4% of participants showed nausea, vomiting, or abdominal pain.
Conclusions
IGB treatment showed good efficacy and safety profile in Korean patients with obesity. In terms of %TBWL and percentage excess body weight loss, the efficacy was similar to that in the Western population.
INTRODUCTION
Obesity is a global pandemic and an established risk factor for various diseases including diabetes, cardiovascular diseases, and cancers.1 The treatment of obesity can be classified as lifestyle modification, pharmacotherapy, endoscopic bariatric and metabolic therapies (EBMTs), and bariatric surgery. Among them, EBMT has drawn attention recently due to its high efficacy and relatively low risk of complications.2,3 Various devices and techniques have been developed so far in the field of EBMT, of which, intragastric balloons (IGBs) are the most popular and well-established.4 Since its first trial in obesity in 1982, various IGBs with different characteristics have been commercialized worldwide.5
Currently, three types of IGBs are available in Korea: End-ball (Endalis), Orbera (Apollo Endosurgery Inc.), and Slim ball (CVBIO Co., Ltd.), which are manufactured and sold by the Korean company CVBIO Co., Ltd. Among these balloons, End-ball is the most popular IGB in Korea. Slim ball is not commonly used, and no clinical studies have been published yet. Orbera balloon has just begun to be sold in Korea after being approved by the Ministry of Food and Drug Safety in March 2018. End-ball was approved by the Ministry of Food and Drug Safety in March 2012, and it has the longest history of clinical use in Korea. However, only a few studies have reported the efficacy and safety of IGB treatment in Korea.6,7 In this study, we aimed to evaluate the efficacy and safety of IGB treatment in Korea.
METHODS
Participants
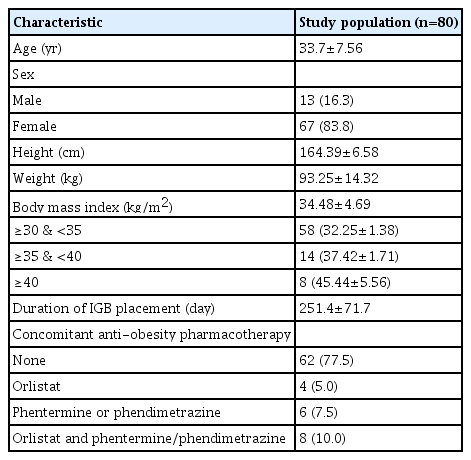
This retrospective cohort study was conducted in a private obesity clinic (We Comfortable Clinic) and an university affiliated hospital (Kangwon National University) from 2013 to 2019 in South Korea. IGB insertion was performed for any participant with a body mass index (BMI) ≥30 kg/m2 who wanted to undergo the procedure after failure of lifestyle modification or pharmacologic interventions. Exclusion criteria were a history of gastric surgery, pregnancy or breast feeding, large hiatal hernia (>5 cm), active peptic ulcers, severe reflux esophagitis (Los Angeles classification grade C or D), Crohn’s disease, alcoholism or drug abuse, severe eating disorders, anticoagulation, and severe liver disease (cirrhosis or hepatic insufficiency).
IGB procedure and patient instructions
Before the insertion of the gastric balloon, esophagogastroduodenoscopy was performed to evaluate upper gastrointestinal pathology. An End-ball was inserted through the esophagus and positioned at the high body of the stomach. Two syringes of air (200 mL) were injected to deploy the balloon and subsequently, 400 to 500 mL of saline was injected using the filling kit. Methylene blue was mixed with the saline to obtain a 1% solution as a color indicator. All procedures were performed under conscious sedation.
After insertion, most acute minor complications, such as nausea and abdominal pain, were managed with antiemetics, antispasmodics, or sedatives for 1 week. The diet was started from a clear liquid diet and slowly increased to a soft diet, and regular diet was introduced slowly during a period of 1 to 2 weeks. After the insertion of the IGB, patients were consulted by a dietitian for appropriate calorie intake and meal preparation. Specifically, instructions and examples of diet plans were provided to the patients, which were composed of 1000–1200 kcal/day sample menus for 2 weeks after the IGB insertion and 1,200 to 1,500 kcal/day thereafter, with the aim of making an energy deficit of at least 500 to 1,000 kcal/day. In addition, the patients were instructed to avoid simple sugars, such as candy, honey, and sweet drinks. Patients were recommended to perform regular exercise for at least 30 min/day. Both aerobic and resistance exercises were appropriately recommended, considering the patients’ exercise capacity.
Patients were regularly followed up monthly during the IGB insertion period and monitored for complications of treatment and compliance with lifestyle modifications. Full-dose dose proton pump inhibitor were continuously prescribed. In addition, patients were instructed to restrict some activities such as diving, flying in an unpressurized aircraft, combat, and extreme sports for possible balloon rupture.
Routinely, the IGB was retrieved 6 months after IGB insertion. Under sedation, the balloon was punctured using a catheter needle, and saline and air were aspirated completely and removed using an extraction hook. After balloon removal, esophagogastroduodenoscopy was performed to detect any possible mucosal damage during extraction.
Anthropometric and outcome parameters
Age, sex, height, body weight, and bioelectrical impedance data at the time of insertion and removal were obtained through medical chart review. BMI (kg/m2), % total body weight loss (%TBWL=(initial weight-post weight)/initial weight×100), and % excess body weight loss (%EWL=(initial weight-post weight)/(initial weight-ideal weight)×100) were calculated. The ideal body weight was defined as a BMI of 25 kg/m2. Minor and major complications during IGB placement were also recorded (i.e., nausea, vomiting, abdominal pain, reflux esophagitis, gastric ulcer, constipation, early IGB removal, spontaneous deflation of IGB, bleeding, perforation, IGB migration, gastric outlet obstruction, and death).
Statistical analysis
A paired t-test was performed to compare the baseline and final outcome parameters. An unpaired t-test was performed to compare weight loss according to adjunctive anti-obesity drug usage status. Statistical significance was set at p<0.05. The NCSS 2022 statistical software (NCSS LLC) was used for statistical analysis.
Ethical statements
The study was approved by the Kangwon National University Hospital Institutional Review Board (IRB No: KWNUH 2019-04-007-009), and was conducted in accordance with ethical standards.
RESULTS
Demographic characteristics
From 2013 to 2019, 86 patients with obesity underwent IGB insertion. Of these, six participants without follow-up anthropometric data were excluded from the analysis, and a total of 80 participants were analyzed in this study. Mean age was 33.7 years and 83.8% of patients were female. Mean BMI was 34.5 kg/m2 at the time of IGB insertion (Table 1). Among the study population, 22 patients (27.5%) showed a BMI larger than 35 kg/m2 (class III or extreme obesity according to the Korean guideline).8 The mean duration of IGB placement was 251.4 days. The majority of the study population (77.5%) did not take adjunctive anti-obesity medications. However, some patients were treated with concomitant anti-obesity pharmacotherapy for a short period of time (1–2 months) (Table 1).
Efficacy and safety of IGB
Table 2 shows body weights and outcome parameters at the time of IGB insertion and removal. Mean body weight change during the IGB insertion was 9.95 kg and %TBWL was 10.76%. Mean BMI loss was 3.72 kg/m2 and mean %EWL was 43.67%. During IGB insertion, significant body weight loss was observed. To evaluate the effect of concomitant anti-obesity pharmacotherapy, we analyzed the efficacy of IGB separately for the 18 patients who took anti-obesity medications during IGB indwelling period and compared the efficacy with patients who did not take anti-obesity medications. There was no significant difference in all outcome parameters between the two groups (Table 3).
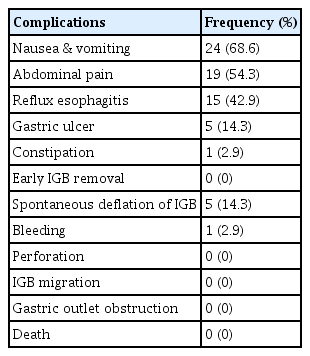
Among 80 patients, data on the complications were available only for 35 participants (35/80, 43.8%). However, we could confirm by electronic medical record review that no major complications (i.e., death or gastrointestinal bleeding/perforation/obstruction leading to hospital admission for endoscopic procedures or surgery) were observed in the remaining 45 participants. Most complications were mild, which were nausea and vomiting (68.6%) and abdominal pain (54.3%), which gradually improved over time (Table 4). No major complications requiring surgery or endoscopic treatment occurred.
DISCUSSION
Despite the global use of IGB, only a few reports with a small number of participants have been published for the Asian population.6,7,9-12 In addition, most Asian studies have reported the efficacy and safety of Orbera (formerly known as the BioEnterics Intragastric Balloon). No data have been reported for End-ball except for two Korean reports.6,7 In this study, we collected and analyzed data on the efficacy and safety of IGB procedure (End-ball) in obese patients in Korea. Mean TBWL was 10.0 kg, mean %TBWL was 10.8%, and mean %EWL was 43.7%, which was comparable to the previous reports (%TBWL 7%–14%, %EWL 24%–50%).13-16 Most patients fulfilled the minimum requirement of weight reduction for EBMT proposed by the American Society for Gastrointestinal Endoscopy (i.e., %EWL ≥25% or total body weight lost ≥5%) during the study period.17
Although IGB treatment is a minimally invasive and reversible procedure with a good safety profile, various complications have been reported to date. These can be classified as adverse events related to the IGB itself and adverse events related to the insertion or removal procedure. Regarding complications of the IGB itself, the most commonly reported adverse events were abdominal pain, nausea, and vomiting occurring in 30% to 60% of patients, which are usually experienced during initial period of IGB placement and improve after accommodation.14,18 Major complications requiring urgent intervention have also been reported. Gastrointestinal ulceration, luminal obstruction, bleeding, and perforation were reported to occur in 0.1% to 2% of patients.14,18 Although rare, mortality cases have been reported. In a Brazilian retrospective study, 12 deaths were reported during the presence of IGB among 41,863 cases (0.03% mortality rate).19 Also, hyperinflation, pancreatitis, and Wernicke–Korsakoff syndrome have been reported.18 In regard to complications related to the insertion or removal procedure, lacerations and perforations can occur typically during removal of IGB at the gastroesophageal junction or upper esophageal sphincter.18 Therefore, endoscopists should be prepared with various IGB withdrawal techniques and endoscopic treatments such as clipping, stenting, and over-the-scope clips.18 Also, the endoscopists should be aware of the possible complications and be prepared to manage them appropriately. Patient selection and avoiding contraindications before IGB placement are important to prevent predictable serious complications. In the present study, the safety of IGB was evaluated. Even though the detailed safety issue was not recorded properly for the majority of the study population (45/80, 56.3%), there were no major adverse events requiring surgery or endoscopic procedures for all 80 participants, which was obvious from the medical records. For the participants with detailed record of safety issues, the most common complications were nausea and vomiting (68.6%), which were addressed with medications, such as antiemetics, antispasmodics, and sedatives for a short period of time. The safety profile showed a tendency similar to that of previous reports.14,18
Pharmacotherapy is less effective than IGB treatment. In terms of %TBWL, pharmacotherapy showed 5% to 8% weight loss compared to 7% to 14% with IGB treatment.14,20 Owing to the safety issues, only four types of medications are available for long-term administration in Korea, which are orlistat, naltrexone-bupropion, liraglutide, and phentermine-topiramate.8 Because of the adverse effects, coexisting diseases should be considered when selecting the drugs. Phentermine is available only for short-term use (less than 3 months) in Korea because of its potential for addiction and the risk of cardiovascular disease. The short-term efficacy of phentermine was reported to be 5.2% in terms of %TBWL after a 12-week treatment.21 However, the efficacy was not durable, and long-term phentermine use was needed to maintain weight loss.22 In the present study, we prescribed anti-obesity medication to a small proportion of patients. The most common prescription was short-term (1–2 months) phentermine/phendimetrazine. Considering the known efficacy of anti-obesity medications and the amount of weight loss (12.1% of %TBWL) observed in the present study in patients who took anti-obesity medications, we assumed that the observed weight loss was mainly due to the effect of IGB treatment. In addition, there was no significant difference in outcome parameters between the patients who received IGB treatment only and those who received IGB treatment plus anti-obesity medications. Few studies have reported the efficacy and safety of combined IGB placement and pharmacotherapy. One study showed no significant difference between IGB/pharmacotherapy versus IGB/lifestyle modifications at 6 months after IGB placement.23 We also observed similar results in the present study, but the small number of patients receiving the combination therapy (n=18) limits the interpretation.
The mean duration of IGB placement in this study (36 weeks) was longer than the usual recommended time for End-ball. Generally, we recommended a 6-month indwelling time. However, some patients wanted to maintain an IGB for longer instead of a 2nd IGB insertion. We usually followed the patients’ opinions for up to 8 to 9 months because we have acknowledged that it is not so dangerous from clinical experience. Some patients could not visit the clinic due to accidents (e.g., ankle fracture) or other personal reasons, and had a very long period of IGB placement (e.g., 455 days). The evidence is not sufficient to clearly define the safety of extended IGB dwelling time; however, several reports have shown the safe removal of IGB up to more than 1 year after insertion.24,25 With regard to efficacy, there seems to be no significant further weight loss compared to standard 6-month removal, even though there is a slight non-significant trend towards greater weight loss.25
This study has some limitations. First, we could not collect data on detailed medical history, except for conditions corresponding to the contraindication of IGB placement. Second, detailed data on the minor complications of IGB were not properly collected for the majority of patients (56.3%). Third, long-term follow-up data after balloon removal were not collected. Therefore, the long-term effects of IGB were not evaluated in this study.
In conclusion, IGB (End-ball) was a safe and effective EBMT for obese patients in Korea. The efficacy and safety profiles were comparable to those of previous reports of the Western obese population in terms of %EWL and %TBWL.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
This study was supported by the 2018 Olympus Korea grant from the Korean Gastrointestinal Endoscopy Research Foundation.
Author Contributions
Conceptualization: SJN, HSC, HLL; Data curation: KGL, SJN; Methodology: HSC, CHP, SHJ; Formal analysis: JHY, KOK, DHK, JWK, WS; Funding acquisition: HLL; Investigation: CHP; Supervision: HSC, HLL; Writing–original draft: KGL, SJN; Writing–review & editing: SJN, JHY, CHP, KOK, DHK, JWK, WS, SHJ.