See commentary "Seeking to understand non-responders to ablative therapy for dysplastic Barrett's esophagus" in Volume 56 on page 180 AbstractBackground/AimsEndoscopic therapy for neoplastic BarrettŌĆÖs esophagus (BE) has become the standard of care over the past two decades. In clinical practice, we regularly encounter patients who fail to achieve complete squamous epithelialization of the esophagus. Although the therapeutic strategies in the individual stages of BE, dysplasia, and esophageal adenocarcinoma are well studied and largely standardized, the problem of inadequate healing after endoscopic therapy is only marginally considered. This study aimed to shed light on the variables influencing inadequate wound healing after endoscopic therapy and the effect of bile acid sequestrants (BAS) on healing.
ResultsIn 12.1% out of 627 patients, insufficient healing was present 8 to 12 weeks after previous endoscopic therapy. The average follow-up duration was 38.8┬▒18.4 months. Complete healing was achieved in 13 patients already after intensifying proton pump inhibitor therapy. Out of 48 patients under BAS, 29 patients (60.4%) showed complete healing. An additional eight patients (16.7%) improved, but only partial healing was achieved. Eleven (22.9%) patients showed no response to BAS augmented therapy.
INTRODUCTIONEndoscopic therapy for neoplastic BarrettŌĆÖs esophagus (BE) has become the standard of care over the past two decades.1,2 This multimodal approach is based on endoscopic resection of early carcinomas, followed by endoscopic ablation of the residual BE, with the aim of preventing metachronous neoplasia. The greatest evidence for ablation exists for radiofrequency ablation (RFA). In addition, argon plasma coagulation (APC) and cryoablation are available as further effective methods.
Nevertheless, in clinical practice, we regularly see patients in whom resection followed by ablation fails to achieve complete squamous epithelialization of the esophagus.3 Thus, we observe inadequate healing with fibrin-covered ulceration, or healing with undesired BarrettŌĆÖs mucosa (neo-BarrettŌĆÖs). Some studies have reported BE recurrence after previous complete endoscopic therapy. Depending on the study design, the so called ŌĆ£neo-BarrettŌĆÖs ŌĆØ is detected again in 4% to 32% of cases.4-6
The aim of complete BE eradication is secondary prevention. The risk reduction for the occurrence of a metachronous neoplasia by complete ablation of the remaining BE after successful endoscopic resection of the degenerated neoplastic lesions has already be shown. Thus, the risk of metachronous neoplasia during the follow-up of remaining BE is approximately 30%.7,8
Although the therapeutic strategies in the individual stages of BE, dysplasia, and esophageal adenocarcinoma (EAC) are well studied and largely standardized, the problem of inadequate healing after endoscopic therapy is only marginally considered.
There is some evidence that, in addition to acidic reflux, duodeno-gastro-esophageal reflux of bile acids also has a synergistic effect on the development of intestinal metaplasia.9 However, it is also known that bile acids do have a cytotoxic effect and influence (shown by an experimental approach mainly for deoxycholic acid) on the inflammation-metaplasia-dysplasia-adenocarcinoma sequence of a BE, as demonstrated by several studies. Bile acids can therefore contribute to the malignant transformation by dysregulating local inflammation and by disturbing control of apoptosis in an already existing BE.10-13
Despite potent acid-suppressing drugs (proton pump inhibitors, PPI), a further increase in EAC has been observed in recent years.14
This study aimed to determine the incidence of inadequate healing after endoscopic therapy for BE. In addition, risk factors for inadequate healing will be presented in comparison with the remaining patient population that did not show similar wound healing disorders. Furthermore, the influence of bile acid sequestrants (BAS) on improvement of healing will be elucidated.
METHODSThe following analysis was based on a retrospective evaluation of endoscopically treated neoplastic BE in a single referral center in Germany. Billing data between 2014ŌĆō2019 were used to identify patients with principal diagnoses coded under the International Classification of Diseases, 10th Revision, German modification (ICD-10GM) C15.4, C15.5, and C16.0. The patient records of the identified cases were systematically reviewed by members of the study team (endoscopically experienced residents or senior physicians). All patients who presented with an esophageal neoplasm between 2014ŌĆō2019 were considered. Follow-up examinations were considered until December 2020. This period corresponds to the time the authors worked at this center.
Neoplastic BE therapy at this high-volume center was highly standardized over the entire period, and the participating practitioners and pathologists were proven experts in diagnostics and therapy.
Treatment protocolPatients were eligible if they had documented BE with histopathologically confirmed low-grade dysplasia, high-grade dysplasia, or mucosal (m1ŌĆōm4) or low risk submucosal (sm1 plus G1-2, L0, V0, R0 basal) adenocarcinoma, and underwent at least one endoscopic therapy session (endoscopy resection, APC, hybrid-APC, RFA or EndoRotor) and at least one follow up endoscopy. Work-up and staging were performed according to the current guideline recommendations. Patients who underwent pharmacological or surgical tumor therapy were not considered. A significant proportion of patients were treated in prospective studies.15-19
Endoscopic treatmentEndoscopic evaluation was performed both natively and by acetic acid chromoendoscopy using high-resolution video endoscopy. Abnormal areas were described according to extent, localization, and biopsy. The areas of mucosal defects were described in terms of size and location. The site pretreated in the therapeutic session based on descriptive and photo documentation was visited and explicitly described with regard to healing or remaining Barrett's mucosa despite previous ablation.
Endoscopically identifiable neoplasms were resected by using the suck-and-cut technique. For more extensive lesions, resection was performed in repeated sessions at intervals of 8 to 12 weeks.
Ablation of the non-neoplastic BE was performed using APC or hybrid-APC (Erbe Elektromedizin GmbH, Tuebingen, Germany), RFA (Medtronic Inc., Minneapolis, MN, USA), or EndoRotor (Interscope Inc., Northbridge, MA, USA) in repeated sessions at intervals of 8 to 12 weeks. The procedure was continued until no visible BE remained (complete endoscopic eradication). If there was any doubt, evaluation was performed visually and was verified by biopsies of the gastroesophageal junction. Tissue samples were systematically processed by a pathologist experienced in the evaluation of EAC.
The diagnosis of residual BE was made endoscopically.
Pharmacological treatmentAfter each endoscopic therapy, all patients were treated with pantoprazole 40 mg orally three times a day for 3 weeks, followed by continuous therapy at a single standard dose (40 mg), or the lowest individual dose achieving complete acid suppression previously determined by pH-metry. If inadequate healing was detected, the pantoprazole intake was checked anamnestically and the dose was increased to three times 40 mg/day, and reevaluation was performed after 8 to 12 weeks. In case of healing with neo-Barrett's, re-ablation of this area was performed followed by pantoprazole 3 times 40 mg/day until the next follow-up in 8 to 12 weeks. If inadequate healing under the maximum dosage of pantoprazole was observed, the maximum acid suppression was continued and cholestyramine 4.5 g three times daily was added.
In case of intolerance to pantoprazole, therapy was performed with esomeprazole. This approach was standardized over the entire period.
Poor healing and missing squamous re-epithelializationPoor healing was defined as continued visible ulceration or fibrin coating in the previously treated area on control endoscopy after 8 to 12 weeks. Missing squamous re-epithelialization was defined as healing with BE mucosa after adequate ablation. Areas in which Ōēź30% of the previously ablated area healed again with BE were included in the evaluation. The estimation was done by the endoscopist and was validated during the processing of the data by means of image documentation. Poor healing and missing squamous re-epithelialization are summarized under the term inadequate healing. An exemplary course with the endoscopic appearance of all manifestations is described here and can be found in Figure 1.
EndpointsThe objective of this study was to determine the incidence of inadequate healing after endoscopic therapy for BE. In addition, risk factors for inadequate healing are presented in comparison with the remaining patient population that did not show similar wound healing disorders. Furthermore, the influence of BAS on improvement of healing will be elucidated.
StatisticsDescriptive statistics are expressed as mean with standard deviation (SD) or as median with interquartile range for nonparametric distribution. Student t-test, Mann-Whitney U-test, chi-square test, and Fisher exact test were used to compare both groups.
In addition, binary logistic regression was performed to estimate the influence of insufficient healing, and multivariate logistic regression was performed to estimate the influence on healing under extended therapy; the regression coefficient and 95% confidence interval (CI) are given. Statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
RESULTSBaselineA total of 1,285 patients presented at least once to our center with histopathologically confirmed esophageal cancer. Of these, 627 met the inclusion criteria mentioned above.
The majority of patients treated were men (85.8%) and had an average age of 64 years. At baseline, 41.3% of patients had short-segment BE, 48.4% long-segment BE (LSBE), and 9.3% ultralong-segment BE (Ōēź8 cm). The average circular BE extension was 3.0 cm, and the average maximum BE extension was 4.9 cm. Hernias were described in 84.2% of patients. Of these, 50.7% were small, 21.5% were medium, and 12.0% were large.
Most patients were treated by APC (67.0%). Furthermore, 5.4% of the included patients were treated by RFA, 4.8% by hybrid-APC, and only 0.6% of the patients were treated by EndoRotor in a pilot series. The average duration of the follow-up was 38.8┬▒18.4 months. The patient characteristics are shown in Table 1.
Cohort with inadequate healingIn 76 (12.1%) of these patients insufficient healing 8 to 12 weeks after previous endoscopic therapy, either endoscopic resection or ablation, could be extrapolated from the patientsŌĆÖ records with a mean overall follow-up of 38.8 months. Correspondingly, the group without evidence of wound healing disorders contained 541 patients. There was no record of endoscopic control for ten patients. The mean age at diagnosis in this cohort was slightly higher than the overall cohort (65.5 years [SD 12.5]).
Male gender significantly predominated both in the group with sufficient healing and in the group with wound healing disorder (83.7% and 92.1%, respectively). The mean age at the time of first endoscopic therapy was 64.0 years compared to 65.5 years in the wound healing disorder group (p=0.34).
Regarding the BE extension before therapy, both groups differed significantly (p<0.001). Thus, in the group with sufficient healing, the average circular expansion of the Barrett's mucosa was 2.4 cm (SD, 3.5), and the average maximum extension was 4.4 cm (SD, 3.5). In the group with wound healing disorders, the respective measurements were 6.5 cm (SD, 3.9) circumferential and 8.2 cm (SD, 3.8) in maximum extension.
The differences become even more apparent when BE lengths were classified. Thus, in the group of patients with wound healing disorders, only 7.9% (n=6) had a short-segment BE (<3 cm), compared to 47.3% (n=253) in the control group. Correspondingly, 51.3% (n=39) and 47.9% (n=258) were distributed to a LSBE (Ōēź3; <8 cm). If we define a maximum BE extension Ōēź8 cm as ultralong-segment BE, 40.8% (n=31) of patients with wound healing disorders could be assigned to this group, as opposed to only 5% (n=27) of patients with regular healing. This observation was highly significant (p<0.001). As BE expansion increases, the risk for inadequate healing increases. For circular expansion the odds ratio (OR) was 4.3 (95% CI, 3.2ŌĆō5.0).
A small hiatal hernia (<3 cm) was documented in 22 of the patients (28.9%) with wound healing disorders vs. 64.9% in the sufficient healing group. A medium hernia (3-5 cm) was present in 24 (31.6% vs. 24.5%) and a large hernia (Ōēź5 cm) in 27 (35.5% vs. 10.6%). In three patients with non-healing BE, no statement regarding the size of hernia could be found in the documentation. In statistical comparisons, the differences among these groups were highly significant (p=0.00013). Significantly more large axial hiatal hernias (n=5, 83.3%) were present in the female subgroup.
In multivariate analysis using a binary logistic model, two of these factors showed a significant influence on healing. The greatest influence was shown by the categorical hernia size. The risk for insufficient healing increased with hernia size (OR, 7.39; 95% CI, 4.42ŌĆō12.30; p<0.001). In addition, the risk for insufficient healing also increased with the maximum BE expansion (OR, 1.46; 95% CI, 1.17ŌĆō1.82; p=0.001). The other variables failed to reach the significance level.
Therapy with BAS for inadequate healingOf a total of 76 patients with wound healing disorders, complete healing was achieved in 13 patients already after intensifying PPI therapy (continuous therapy, 3├Ś40 mg PPI for at least 8 to 12 weeks). In our cohort, four patients stated that they had not taken the medication regularly. In one patient, ulcerations were seen as a consequence of increased non-steroidal anti-inflammatory drug intake. Two patients had opiates in their long-term medication. In one patient, the esophageal passage was obstructed by a Zenker diverticulum.
In the remaining 54 patients, intensified acid suppression with maximal PPI therapy and prolonged therapy intervals (doubled) was unsuccessful, and therapy extension with BAS was implemented. Primarily, cholestyramine 4.5 g three times daily was prescribed.
In 15 patients, no data were available after initiation of therapy. Out of 48 patients with documented course of therapy, 29 patients (60.4%) under BAS showed complete healing, which in 24 patients (50.0%) persisted during the entire follow-up period. In the other five patients (10.4%), only partial healing was observed in the further course. In eight patients (16.7%) partial healing was documented over the entire course of therapy, and in 11 (22.9%) no healing was observed at all. Three patients (6.3%) reported cholestyramine intolerance, and two were switched to ursodeoxycholic acid and Lipocol and healed completely during the course. One patient with cholestyramine intolerance underwent esophagectomy because of oncologic reasons.
In the multivariate analysis, which must be viewed with increased caution due to the small number of cases, female gender was the only statistically significant protective factor with an OR for male sex of 18.5 (p<0.0001 for 95% CI). The other parameters clearly failed to reach the significance level. Figure 2 summarizes the healing results according to the therapy in a flow chart.
Further courseIn one out of 24 patients who healed completely after addition of BAS to the therapeutic regimen, esophagectomy was performed for oncological reasons. In one patient an EndoStim system (EndoStim; EndoStim BV, Nijmegen, The Netherlands) was implanted during the endoscopic therapy period; subsequently, a sufficient healing was observed. However, it cannot be determined with certainty which of the therapies contributed most to the healing. After sufficient healing, two patients underwent fundoplication.
Out of 12 patients with partial healing over the entire course, eight underwent fundoplication. Of these, one had already been created in advance and was insufficient at the time of diagnosis. However, healing was not sufficient even after re-fundoplication. Two patients underwent hiatoplasty with fundoplication for (partial) thoracic stomach. Another patient had to undergo esophagectomy for unresectable BE neoplasia. In one patient, fundoplication was insufficient, so a Roux-Y bypass was performed as ultima-ratio. Two patients had severe and ongoing alcoholism.
In the group (n=11) with no tendency to heal even after BAS therapy, two esophageal resections were performed, and three patients underwent fundoplication.
DISCUSSIONWe observed wound healing disorders in almost every 8th patient in our cohort, which is in concordance with previous and recently published studies.3,20 That means that a non-healing BE after endoscopic therapy is a frequent problem and must be considered by interventional endoscopists. The definition of complete eradication of intestinal metaplasia varies. In some studies, the duration or the number of sessions is used to evaluate the success of eradication. From our point of view, this does not appear to be purposeful since, on the one hand, the remaining, dysplasia-free area of the BE after endoscopic resection varies, and on the other hand, wound healing disorders can already manifest themselves after the first therapeutic session as a lack of squamous cell epithelialization of the previously treated area.
Furthermore, in contrast to most of the available data, therapy at our center was predominantly carried out using APC. However, no significant differences were found between ablation methods in terms of insufficient healing.16,21
One important finding is that under intensified PPI therapy, we were able to achieve complete and sustained healing in 13 patients (17.1%). If necessary, an escalation up to the maximum PPI dosage should be undertaken. Here, the endoscopist must specifically address fears of long-term PPI therapy and, in the patient's best interest, provide the necessary information to ensure good compliance. Possible reasons for a lack of response under intensified PPI therapy could be insufficient compliance, concomitant medication, or a resorption disorder. In most patients, after successful healing the PPI dose could be reduced to 1 to 2 standard doses of PPI per day, in order to maintain the squamous epithelialization.
With an OR of 7.39 our data support a major influence of hernia size on inadequate healing. Furthermore, a large hiatal hernia can be postulated as a negative predictor for successful BAS therapy. In our group, a large axial hiatal hernia was present in almost two thirds of the cases with no improvement in healing under BAS. This observation is consistent with previous publications and may be considered a generally accepted risk factor for both the development of BE and its complications.22 We have observed the development of carcinomas and wound healing disorders after endoscopic therapy even after successful fundoplication. In our study the available data on patients who underwent antireflux surgery are inconsistent with a very small number of cases. However, a cohort study from five northern countries could not show any influence of antireflux surgery on the development of adenocarcinomas of the esophagus.23
If an escalation of acid suppressive therapy did not lead to complete healing, we could show the effect of BAS. In 77.1% of the cases, improved squamous cell healing was documented, which was complete and permanent in 50.0% of the cases examined. In 10.4% of cases wound healing disorders were observed again in the further course. Data from a recently published double-blind study on BAS with an optimized colesevelam formulation, showed an improvement in reflux symptoms with good tolerability and can support our conclusion.24 However, further studies are necessary to evaluate the role of BAS in the context of gastroesophageal reflux disease or BE. Impedance-pH-metry (MII-pH) was not used in our study, due to a lack of validation of the measurement in BE. The experience gained in our center with impedance-pH-metry in BE patients shows unsatisfactory results, which are due to a different impedance of the squamous and columnar epithelium. This issue allows to detect a BE by impedance measurements.25 In order to make these differences clinically useful, our scientific group has carried out a series of measurements on esophageal specimens.26
In the multivariate analysis we could show a negative influence of BE length on wound healing (OR, 1.46). In consistence with this, it has already been demonstrated that BE length has an influence on risk of malignant transformation.27 BE length, however, does not appear to be an entirely independent factor, but seems to depend on the size of the axial hernia.28 Unfortunately, other possible influencing variables such as body mass index and smoking could not be adequately evaluated from the patient records.
Due to the retrospective nature of our analysis, the validity of this study is methodologically limited. The procedure at our center for the therapy of BE neoplasia was strictly standardized over the entire observation period and at least the endoscopic and pathologic parameters were prospectively recorded. This somewhat limits the restrictions that result from the study design.
However, a retrospective evaluation only allows limited conclusions to be drawn about the patients' intake modalities. At discharge, the patient was given strict recommendations on PPI intake, both verbally and in writing. In the case of wound healing disorders, it was obligatory to take a medical history and, in case of doubt, to emphasize the importance of antireflux therapy. Before initiating BAS therapy, it was ensured that consistent and maximal PPI therapy was implemented.
In cases of insufficient healing even under exhaustion of PPI, treatment with BAS can be an option as an ultimate healing attempt. Intervention studies addressing this question are needed. Esophageal resection should only be performed for oncological reasons, but not for treating a non-healing, dysplasia-free BE.
NOTESAuthor Contributions
Conceptualization: LW, AM, MK; Data curation: LW, TB, JW, EM, MH, MK; Formal analysis: LW; Investigation: AM, JW, MK; Methodology: LW, MK; Project administration: MK; Resources: AM, MFR, MK; Supervision: AM, MK; Visualization: LW; WritingŌĆōoriginal draft: LW, TB, EM, MH; WritingŌĆōreview & editing: JW, AM, MFR, MK.
Fig.┬Ā1.Endoscopic course in one patient. (A) Mucosal adenocarcinoma before the start of therapy (arrow). (B) Fibrin-covered poor healing after endoscopic resection (whitish lesion at 9 o'clock). (C) Healing with neo-BarrettŌĆÖs (reddish tongue-like area, arrow PE2) under proton pump inhibitor (PPI) and cholestyramine therapy. (D) Complete healing after thermic ablation with PPI and cholestyramine therapy. 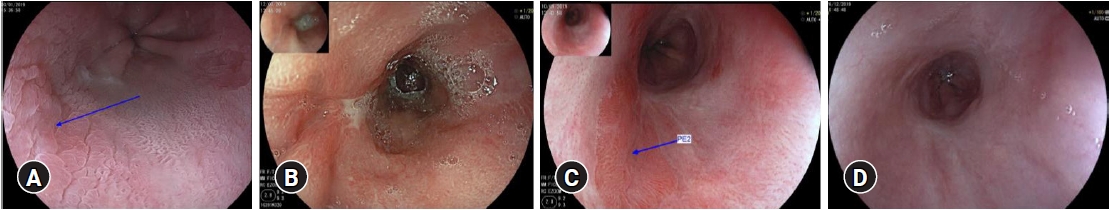
Fig.┬Ā2.A Flowchart showing patients included in this study. ICD-10GM, International Classification of Diseases, 10th Revision, German modification; AC, adenocarcinoma; SCC, squamous cell carcinoma; PPI, proton pump inhibitor; BAS, bile acid sequestrants. 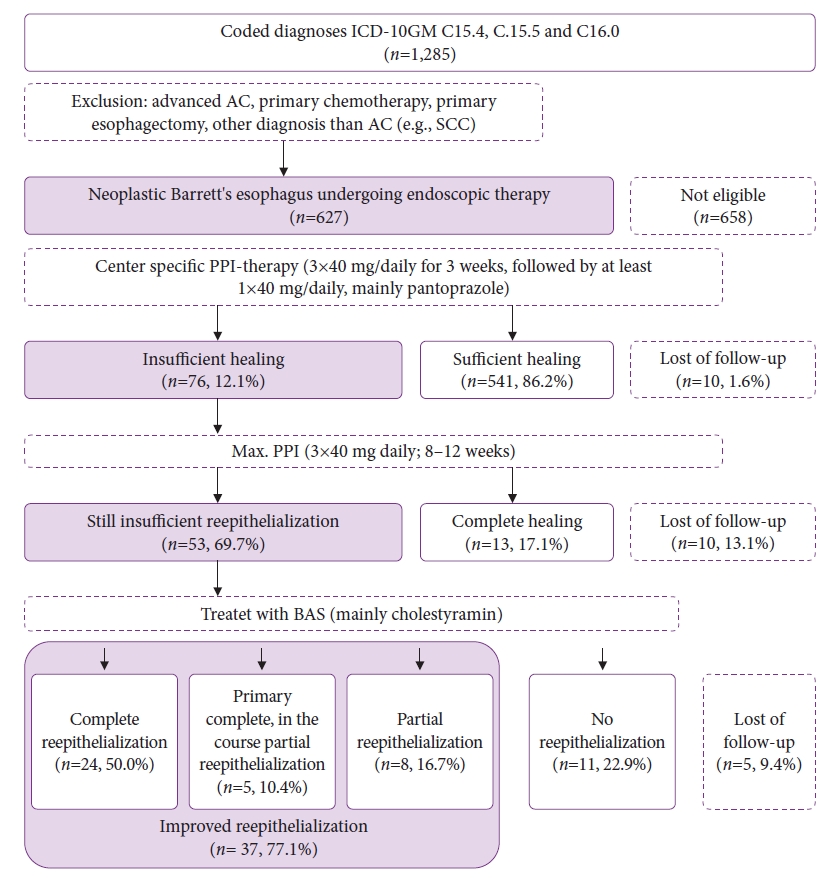
Table┬Ā1.Patient characteristics Values are presented as mean┬▒standard deviation or number (%). BE, BarrettŌĆÖs esophagus; SSBE, short-segment BE; LSBE, long-segment BE; ULSBE, ultralong-segment BE; LGIN, low-grade intraepithelial neoplasia; HGIN, high-grade intraepithelial neoplasia; m, mucosal (m1ŌĆōm4); sm, submucosal (sm1ŌĆōsm3); APC, argon plasma coagulation; RFA, radiofrequency ablation. REFERENCES1. Standards of Practice Committee, Wani S, Qumseya B, et al. Endoscopic eradication therapy for patients with Barrett's esophagus-associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907ŌĆō931.
2. Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2017;49:191ŌĆō198.
3. van Munster SN, Frederiks CN, Nieuwenhuis EA, et al. Incidence and outcomes of poor healing and poor squamous regeneration after radiofrequency ablation therapy for early Barrett's neoplasia. Endoscopy 2022;54:229ŌĆō240.
4. Tan MC, Kanthasamy KA, Yeh AG, et al. Factors associated with recurrence of Barrett's esophagus after radiofrequency ablation. Clin Gastroenterol Hepatol 2019;17:65ŌĆō72.
5. Cotton CC, Wolf WA, Overholt BF, et al. Late recurrence of Barrett's esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in intestinal metaplasia containing dysplasia trial. Gastroenterology 2017;153:681ŌĆō688.
6. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209ŌĆō1217.
7. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14:1085ŌĆō1091.
8. Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6ŌĆō12.
9. Oh DS, Demeester SR. Pathophysiology and treatment of Barrett's esophagus. World J Gastroenterol 2010;16:3762ŌĆō3772.
10. Huo X, Juergens S, Zhang X, et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-╬║B activation in benign Barrett's epithelial cells. Am J Physiol Gastrointest Liver Physiol 2011;301:G278ŌĆō86.
11. Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012;21:36ŌĆō51.
12. Matsuzaki J, Suzuki H, Tsugawa H, et al. Bile acids increase levels of microRNAs 221 and 222, leading to degradation of CDX2 during esophageal carcinogenesis. Gastroenterology 2013;145:1300ŌĆō1311.
13. O'Riordan JM, Abdel-latif MM, Ravi N, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol 2005;100:1257ŌĆō1264.
14. Cattelan L, Ghazawi FM, Le M, et al. Epidemiologic trends and geographic distribution of esophageal cancer in Canada: a national population-based study. Cancer Med 2020;9:401ŌĆō417.
15. Knabe M, Beyna T, R├Čsch T, et al. Hybrid APC in combination with resection for the endoscopic treatment of neoplastic Barrett's esophagus: a prospective, multicenter study. Am J Gastroenterol 2022;117:110ŌĆō119.
16. Knabe M, Wetzka J, Kronsbein H, et al. Hybrid argon-plasma-koagulation versus radiofrequenzablation nach endoskopischer resektion neoplastischer l├żsionen im Barrett├Čsophagus. Eine randomisierte studie an einem terti├żren zentrum. Z Gastroenterol 2020;58:e142.
17. Knabe M, Bl├Č├¤er S, Wetzka J, et al. Non-thermal ablation of non-neoplastic Barrett's esophagus with the novel EndoRotor┬« resection device. United European Gastroenterol J 2018;6:678ŌĆō683.
18. Knabe M, Welsch L, Blasberg T, et al. Artificial intelligence-assisted staging in Barrett's carcinoma. Endoscopy 2022;54:1191ŌĆō1197.
19. Knabe M, Beyna T, R├Čsch T, et al. Hybrid APC in combination with resection for the endoscopic treatment of neoplastic Barrett's esophagus: a prospective, multicenter study. Am J Gastroenterol 2022;117:110ŌĆō119.
20. Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652ŌĆō660.
21. Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2019;89:680ŌĆō689.
22. Kwon JY, Kesler AM, Wolfsen HC, et al. Hiatal hernia associated with higher odds of dysplasia in patients with Barrett's esophagus. Dig Dis Sci 2021;66:2717ŌĆō2723.
23. Maret-Ouda J, Santoni G, Wahlin K, et al. Esophageal adenocarcinoma after antireflux surgery in a cohort study from the 5 Nordic countries. Ann Surg 2021;274:e535ŌĆōe540.
24. Vaezi MF, Fass R, Vakil N, et al. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a randomized trial. Gastroenterology 2020;158:2093ŌĆō2103.
25. Kataria R, Rosenfeld B, Malik Z, et al. Distal esophageal impedance measured by high-resolution esophageal manometry with impedance suggests the presence of Barrett's esophagus. J Neurogastroenterol Motil 2020;26:344ŌĆō351.
26. Bl├Č├¤er S, May A, Welsch L, et al. Virtual biopsy by electrical impedance spectroscopy in Barrett's carcinoma. J Gastrointest Cancer 2022;53:948ŌĆō957.
|
|
||||||||||||||||||||||||||||||||||||||||||||
