INTRODUCTION
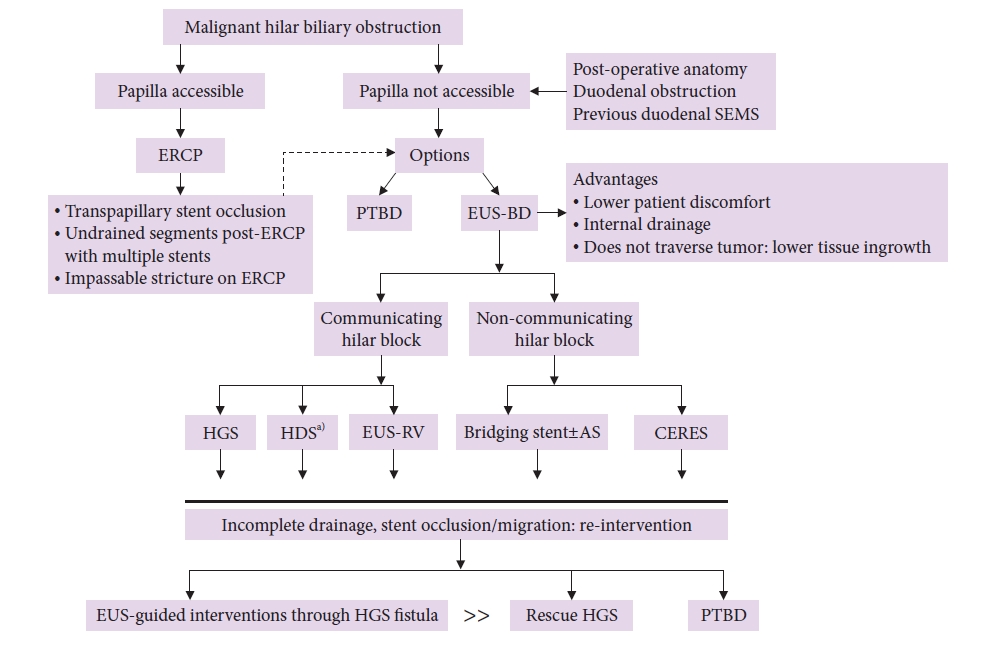
Biliary drainage in advanced malignant hilar biliary obstruction (MHBO) is challenging, particularly in Bismuth III- and IV-type blocks. Complex MHBO requires the drainage of multiple liver segments to achieve clinical success. Bilateral stenting by endoscopic retrograde cholangiopancreatography (ERCP) is preferred over unilateral stenting to drain the maximum volume of the liver. Although the risk of cholangitis increases with the complexity of the hilar block,1 ERCP may not be possible due to inaccessible papilla in certain situations, for example, surgically altered anatomy, duodenal narrowing, or prior duodenal self-expanding metal stent (SEMS) for duodenal obstruction. Percutaneous trans-hepatic biliary drainage (PTBD) and EUS-BD are the routes available in such scenarios. These alternate drainage options may also be considered as re-intervention for failed ERCP in cases of occluded transpapillary stents. EUS-BD is preferred over PTBD for MHBO with inaccessible papilla owing to less patient discomfort, internal route of drainage, avoidance of traversing the stricture, and biliary drainage distant from the tumor with a lower probability of tissue ingrowth causing stent occlusion.2
The common options for EUS-BD in MHBO and inaccessible papilla include (1) EUS-guided hepatico-gastrostomy (EUS-HGS) (Fig. 1), (2) EUS-guided hepatico-duodenostomy (EUS-HDS), and (3) EUS-guided antegrade stenting (EUS-AS). Other occasionally used methods include (1) the bridging method (HGS/HDS with bridging stent connecting right to left duct), (2) combined ERCP and EUS-BD (CERES): EUS-HDS with ERCP for left side biliary stenting or EUS-HGS with ERCP for right side biliary stenting, and (3) EUS-guided rendezvous.3
EUS-HGS, or occasionally EUS-HDS, can suffice for bilateral communication systems in MHBO (i.e., Bismuth type I). EUS-rendezvous is an option in this scenario but may not be feasible in those with inaccessible papillae, even though SEMS placement has been described with colonoscopy to reach inaccessible papillae after EUS-rendezvous.4 For non-communicating systems, bridging stents or CERES are options. Single-session CERES may not be feasible for inaccessible papilla; however, it can be used for re-intervention after initial ERCP if additional duct drainage is required, which is infeasible with ERCP (e.g., due to duodenal obstruction).5 EUS-AS has been used for hepaticojejunostomy stricture. Rendezvous and additional stent placement via colonoscopy, re-intervention for blocked HGS/HDS stents, bridging stents for non-communicating systems, and the multi-stent technique.4,6,7
We shall discuss the currently available literature on EUS-guided interventions for inaccessible papillae in the setting of MHBO.
EVOLUTION OF EUS-GUIDED INTERVENTIONS FOR MHBO WITH INACCESSIBLE PAPILLA
The first case of palliative HGS was performed by Giovannini et al.8 in 2003, in which ERCP was not possible because of prior subtotal gastrectomy for gastric adenocarcinoma. Echo-endoscope-guided left hepatic duct puncture was performed using a 19-G needle, followed by an initial plastic stent and an 8 cm long covered metal stent placement. Later in 2007, the same French group published their experience with 11 patients (8 malignant) in whom ERCP was not possible due to duodenal narrowing, surgically altered anatomy, or left hilar obstruction. EUS-HGS was performed in 10 of 11 patients (failed guidewire insertion in one). Of the seven patients who underwent plastic stent placement, one had early stent occlusion and one had ileus. Among the three patients who received covered metal stents, one had biloma and one had cholangitis related to stent shortening. However, clinical success was achieved in all the ten patients. On follow-up, two patients had stent occlusion due to tissue ingrowth and one had stent migration; all were treated endoscopically with a second uncovered metallic stent.9
The Korean group reported five cases of EUS-HGS and fully covered (FC) stent placement for occluded biliary metal stent placement prior to ERCP (bilateral hilar stenting or combined biliary and duodenal stenting).10 The clinical success rate was 100% without any complications (migration/occlusion) or re-interventions over a median follow-up of five months. Hence, it was concluded that EUS-HGS is a good alternative to PTBD for occluded biliary stents after failed ERCP.
Two cases of hilar cholangiocarcinoma (one with Roux-en-Y anatomy and the other with left hilar obstruction after right duct stenting) treated with EUS-HGS was reported in 2011.11 Both cases showed clinical success, as evidenced by decrease in bilirubin levels. The first patient died after one month due to sepsis and liver failure, and the stent was positioned near the esophagogastric junction. The second patient survived for six months. It was concluded that EUS-HGS is a feasible and safe modality with the advantage of internal drainage far away from tumor-promoting hastened recovery. The same group reported their experience of over 4 years in ten patients, with technical and clinical success rates of 80% and 70%, respectively.12 One ineffective drainage was due to stent malposition, and in two cases, stents migrated, which were managed endoscopically by the placement of additional metal and plastic stents.9
A Japanese study reported the clinical usefulness of EUS-BD in high-grade hilar obstruction with failed re-intervention by ERCP, in which ten patients underwent EUS-BD.13 Technical success was 100%, with similar stent patency compared to re-intervention with ERCP.
BILATERAL BILIARY DRAINAGE WITH EUS-HGS
One of the major limitations of EUS-HGS for malignant hilar obstruction is the ability to drain only the left hepatic duct. In 2014, Ogura et al.14 described a case of hilar obstruction due to colorectal carcinoma metastasis, treated with two sequentially placed metallic stents (first uncovered 8 mm stent connecting the left to right hepatic duct and second 10 mm covered stent used for HGS) (Table 1). Reim├Żo et al.15 reported bilateral biliary drainage using EUS-HGS in nine MHBO cases in the same year. They described a 3-step procedure consisting of puncture of the left hepatic duct with a 19-G needle followed by insertion of a 0.035-inch guidewire into the right hepatic duct, followed by dilation with a 6-Fr cystotome. Later, an uncovered SEMS was placed, bridging the right and left hepatic ducts and another partially covered stent for hepaticogastrostomy. Technical and clinical success was achieved in seven cases; two developed complications within 72 hours: one had drainage failure and another developed sepsis and died later. Chemotherapy was successfully initiated in six patients (Table 1). A recent study by a French group showed that the technical and clinical success rates of the bridging technique with uncovered SEMS for hilar stenosis were 100% and 83%, respectively.16 Chemotherapy was initiated in 70% of patients. Adverse events occurred in one-third of the patients, but only one patient required salvage PTBD. Mortality due to fulminant sepsis occurred in 8% of patients, with a median survival of six months (Table 1). Similarly, some case reports showed bilateral system drainage using the left hepatic duct approach.17
In 2016, EUS-guided drainage of the right biliary system was performed using a dedicated plastic stent (7 Fr) after puncturing the right hepatic duct from the duodenal bulb.18 Various techniques have been described for bilateral drainage. A novel approach for simultaneous HGS (left hepatic duct from the stomach) and HDS (right hepatic duct from the first part of the duodenum) has been described for bilateral biliary drainage for treating recurrent hilar obstruction after multi-stent placement via PTBD.19 Transverse bridging stent placement through an HGS stent can help manage MHBO, as EUS-HDS can be technically challenging.20
EUS-HDS IN SEGREGATED RIGHT INTRA-HEPATIC DUCT
Ogura et al.21 described 11 cases in which EUS-BD was performed for right hepatic duct obstruction in 2015. Four patients underwent HDS, and the remaining seven underwent bridging stent placement. EUS-HDS for segregated right hepatic duct was described in a retrospective study from Korea in 35 patients (71.4% malignant etiology). Technical success was 97.1%, and clinical success was 80%. Approximately 20% of patients experienced adverse events. EUS-HDS for segregated right posterior hepatic duct was associated with significantly higher 3-month stent patency rates (79.1%) than for the right anterior hepatic duct (38.1%) (Table 1).22
RE-INTERVENTION AFTER EUS-BD
Re-intervention after EUS-HGS may be required after incomplete drainage, stent occlusion, or stent migration. In 2018, after EUS-HGS with plastic stent failed, Uchida et al.23 reported re-intervention using two metallic stents (one uncovered bridging the right and left hepatic duct and the second as an HGS stent) and a multi-path occlusion balloon (Bouncer; Cook Medical, Tokyo, Japan) with a multi-lumen located on either side of the balloon, thus facilitating the passage for guidewires into both the right hepatic duct and downstream common bile duct from the left hepatic duct.
Re-intervention after EUS-HGS with FC-SEMS can be performed by cannulation through the SEMS or argon plasma coagulation-guided trimming of the SEMS. However, to penetrate the covered part of the HGS SEMS, a simplified method using a diathermic dilator (Cysto-Gastro-Set; Endo-Flex GmbH, Voerde, Germany) with an electrosurgical generator was used. Re-intervention by covered SEMS was used for blocked antegrade stents and uncovered SEMS for blocked HGS stents.6
Re-intervention can be performed easily with short FC-SEMS (6 mm) compared to large bore stents, which tend to cause more tissue hyperplasia, making stent removal difficult. The reported technical success rate of EUS-HGS with this short stent was 100%, with a clinical success rate of 95% in a study of 20 patients. Adverse events occurred in 15% of patients, and stent dysfunction requiring re-intervention was successful in nine out of ten patients (90%). One patient required PTBD for stent dysfunction.24
Recurrent biliary obstruction (RBO) can occur in one-third of patients after placement of a long (8ŌĆō12 cm) partially covered SEMS (modified Giobor stent; Taewoong Medical, Seoul, Korea) via EUS-HGS for malignant biliary obstruction. Short intragastric stent length and prior drainage were factors associated with RBO. However, re-intervention was possible in 90% of the cases via the HGS route. PTBD and rescue EUS-HGS were required in the remaining patients.25
GUIDEWIRE MANIPULATION
Puncturing the left hepatic duct at a proper angle is essential for directing the guidewire towards the appropriate ducts. A novel method using an uneven double-lumen cannula (Piolax Medical Devices, Kanagawa, Japan) was used to manipulate guidewire passage into the right hepatic duct, which was initially impossible.26 The first guidewire prevents entry of the second guidewire into the undesired duct and guide advancement of the second guidewire into the desired duct.27
CERES
CERES is particularly helpful for MHBO, overcoming the disadvantages of both PTBD (external drainage), and ERCP with bilateral SEMS placement (technically challenging). For ERCP with SEMS placement on the right side and a non-functioning right lobe of the liver, EUS-HGS can be performed. Similarly, EUS-HDS can be performed for ERCP with SEMS placement on the left side and non-functioning left lobe of the liver. Two studies published in 2017 showed the safety and efficacy of EUS-BD in MHBO, in which ERCP either failed or was infeasible (altered anatomy/duodenal stenosis/prior duodenal stenting), or additional interventions were indicated for adequate biliary drainage.5,28 The first study by Minaga et al.5 reported technical and clinical success rates of 97% and 76%, respectively. Mild peritonitis and stent dysfunction were reported in 10% and 23.3%, respectively. Another study by Moryoussef et al.28 reported technical and clinical success rates of 94% and 72.2%, respectively. Complications and re-interventions occurred in 16.7% of patients. A recent study by a French group reported technical and clinical success rates of 95% and 100%, respectively.29 Among the 20 patients with inoperable MHBO in whom EUS-BD was used as the initial procedure or re-intervention, CERES was performed in 11 cases (5 single sessions, 6 required two sessions). The early complication and mortality rates were 35% (bile leak, fecaloma, cholangitis, and pulmonary embolism) and 5%, respectively (Table 1).29
A case report describes a hepaticojejunostomy anastomotic narrowing caused by hepatic mass after pancreaticoduodenectomy, which was treated with EUS-AS with uncovered SEMS, followed by the placement of a guidewire through which a second metal stent was placed using the stent-in-stent technique by colonoscopy.4
RE-INTERVENTION AFTER MULTIPLE METALLIC STENTS PLACEMENT FOR MHBO
Re-intervention is difficult after the placement of multiple hilar metallic stents for MHBO, more so if stent-in-stent formation is used rather than side-by-side stent placement.30 EUS-BD has been compared with transpapillary endoscopic re-intervention in a retrospective study of MHBO treated with multiple metallic stents. In all 15 cases where transpapillary intervention failed, EUS-guided re-intervention was successful with 86.7% clinical success rate. The time to RBO was significantly longer with EUS-BD (212 days) as compared to transpapillary re-intervention (84 days). Biliary per-itonitis occurred in 13.3% of cases that were managed conservatively (Table 1).31
A case of recurrent hilar biliary obstruction after placing bilateral uncovered SEMS with cholangitis in the right posterior hepatic duct was described using EUS-HDS-guided antegrade stenting with uncovered SEMS and FC-SEMS for HDS fistula.32 Molting technique using a novel stent delivery system with a dilation function (EndoSheather; Piolax Medical Devices) was shown to be useful in 12 patients with MHBO after multiple uncovered self expanding metal stents deployments (Table 1).33
CERES VERSUS BILATERAL PTBD FOR MHBO
CERES can provide a lower rate of RBO (27%) compared to bilateral PTBD (88%), with similar complication and mortality rates in MHBO. The median time to RBO was 92 days with CERES and 40 days with PTBD. This is because of the distance between the biliary drainage site and the tumor, thus avoiding tumor ingrowth (Table 1).5,9,12,14,16,21,22,28,29,31,33,34 In practice, EUS-BD in the same session as ERCP is warranted if the injected bile duct segments cannot be drained using ERCP.35
STENT-IN-STENT AND MULTI-STENT METHOD
The stent-in-stent method has been described using EUS-BD for MHBO. Due to the lack of reduction in bilirubin level after EUS-guided antegrade metal stent placement in the B2 segment, a second stent was placed using the stent-in-stent technique in the B3 segment.7 In another case report, after placing a SEMS bridging the right and left hepatic ducts, another SEMS was placed through the first in the lower common bile duct to the left hepatic duct.36
EUS-HGS-guided bridging stent placement in the hilum with a novel SEMS and 6-Fr delivery system (NitiŌĆÉS large-cell SR slim delivery; Taewoong Medical) has been described, followed by antegrade stenting using the stent-in-stent technique after balloon dilation through the stent mesh.37
Recently, two covered metal stent placements have been reported for separated right anterior and posterior hepatic ducts after EUS-HGS with a plastic stent in the left hepatic duct.38
NOVEL INTERVENTIONS THROUGH EUS-GUIDED HGS
Biliary interventions, such as photodynamic therapy and argon plasma coagulation, can be performed using EUS-HGS FC-SEMS. Tumor palliation and bleeding control can also be achieved.39
EUS-BD IN CONJUNCTION WITH INTERVENTIONAL RADIOLOGY
EUS-BD can be performed more effectively in conjunction with interventional radiology. In a case of high-grade biliary obstruction with blocked stents placed through PTBD, three stents were placed using EUS-HGS under the guidance of interventional radiology. The guidewire passed through the HGS was captured through the PTBD route, and a balloon expandable stent (10 mm) was placed across the hilum, followed by a HGS stent and a third stent between the hilar bridging and HGS stents (both 8 mm).40
PROCEDURAL CONSIDERATIONS OF EUS-GUIDED INTERVENTIONS IN MHBO
Proper case selection is the first step in EUS intervention for MHBO. MHBO with inaccessible papilla due to surgically altered anatomy and duodenal obstruction is a candidate for EUS-BD as an alternative to PTBD. Since the presence of ascites is a relative contraindication, large ascites should be drained percutaneously before EUS-BD. Rapid filling of ascites can increase the physical gap between the stomach and the liver, leading to stent displacement and peritonitis. Left lobe atrophy is a contraindication for left duct EUS-HGS. Tumor infiltration of the stomach at the puncture site is also a contraindication. Therefore, caution should be exercised in cases of unresectable gastric cancer with reduced stomach volume due to the high risk of stent migration. Despite the technical feasibility of EUS-HGS, the isolated right hepatic duct should be drained using EUS-HDS.41
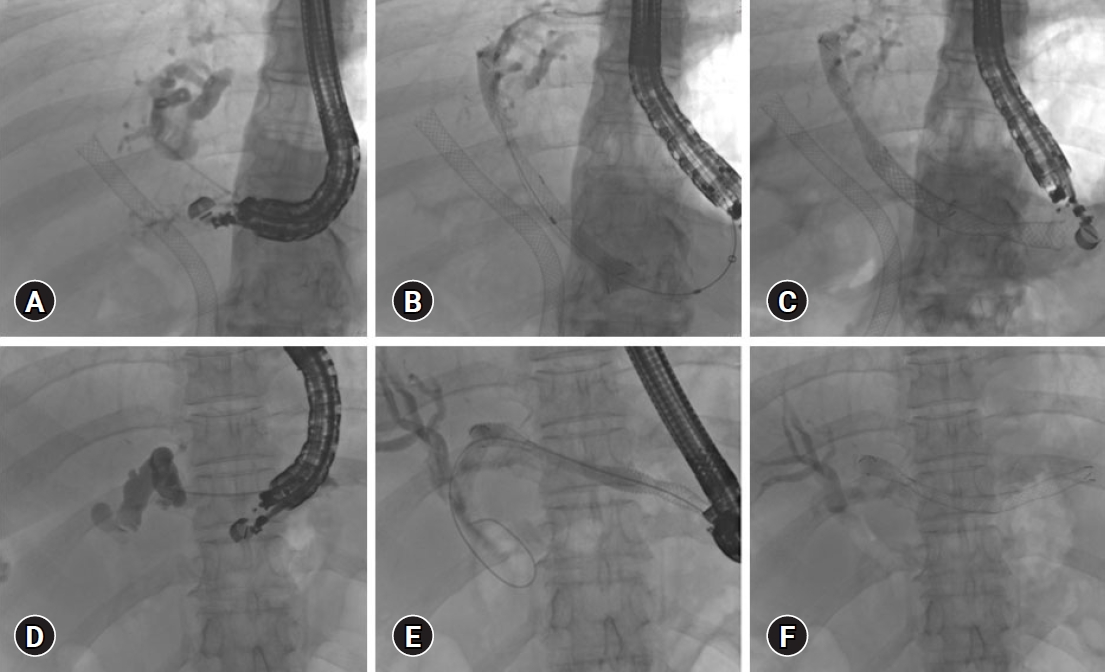
The second step is puncture site selection (Fig. 2A). Although guidewire manipulation is easier through segment 2 due to its straight course, the segment 3 duct is preferred over the segment 2 duct puncture. However, a risk of mediastinitis with segment 2 puncture exists due to frequent access to the esophagus. The needle should be directed towards the hilum of the liver. A puncture perpendicular to the duct makes guidewire manipulation more difficult. An angulated scope position may lead to an unstable scope position and a reduced forward force in the device, causing looping and accessory displacement.41
The next step is the needle and guidewire selection. A 19-G needle is preferred for dilated bile ducts, whereas a 22-G needle is preferred for non-dilated bile ducts. A stiff guidewire with a flexible tip is preferred. A 0.035-inch guidewire passes easily through a 19-G needle but may shear on excessive pull and push through the sharp end, which can be circumvented using a hydrophilic 0.025-inch stiff guidewire (Fig. 2B). For the 22-G needle puncture, a stiff 0.021- or 0.018-inch guidewire is used.41
Tract dilation is usually performed with a coaxial electrocautery dilator, but bleeding from the fibrotic liver is a concern. To avoid multistep stent deployment, various modifications, such as thin-tip balloon catheters with or without a stylet, metallic stents with a thin delivery system, or electrocautery at the tip, are being used.41
Stent selection is the final step. FC-SEMS (Fig. 2CŌĆōF) is preferred over plastic stents because of its larger diameter and lower need for re-intervention. However, FC-SEMS can cause blockage of the intra-hepatic side branches. Hence, a partially covered SEMS with an uncovered segment positioned in the intra-hepatic duct, a covered segment positioned between the liver and stomach, and a flared end in the stomach has been developed.41 A smaller caliber (6 mm) FC-SEMS can potentially reduce the risk of side-branch blockage and biliary hyperplasia. A dedicated single-pigtail plastic stent for interventional EUS with anti-migration properties (four flanges and a tapered tip) has been developed, although non-availability outside Japan and repeated re-interventions are the drawbacks.24
COMPLICATIONS
Complications associated with EUS-BD include vascular injury (portal vein/hepatic artery), bile leak and biloma, liver abscess, cholangitis, pseudoaneurysm, and hyperplasia-induced stent obstruction. In most cases, a pseudoaneurysm caused by vascular injury to the hepatic artery can be treated transarterially. The probability of bile leak was higher with the placement of plastic stents alone, with >1 puncture, longer procedure time (>20 minutes), acute cholangitis, and shorter distance to liver parenchyma (<2.5 cm). An FC-SEMS between the liver and stomach can prevent bile leaks.41
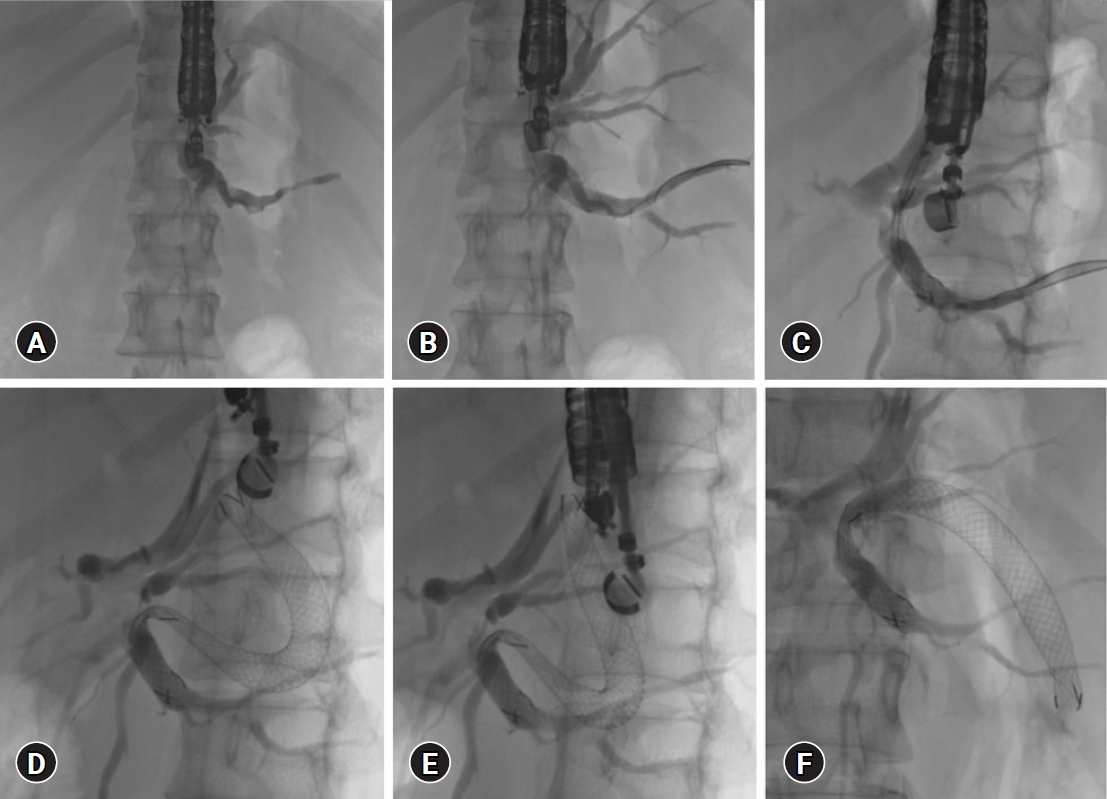
Immediate or delayed stent migration can occur. Deployment of the stent within the scope (Fig. 2C) and keeping >3 cm on the gastric side helped prevent stent migration. Intra-procedural stent migration can be managed by retrieving a misplaced stent or placing another stent. Delayed migration can be prevented by using partially covered stents and plastic stents within the metal stents (Fig. 3AŌĆōC) and the use of both covered and uncovered stents to secure the intra-hepatic portion.41
Stent migration (proximal gastric end) into the abdominal cavity has been reported after EUS-guided HG is performed for malignant hilar obstruction due to hepatocellular carcinoma. Laparotomy was performed in the same manner, and the stent was reinserted into the fistula and sutured to prevent remigration.42
Stent occlusion was more common after EUS-HGS was performed using plastic stents (median patency: 108 days) than after SEMS (median patency: 158 days). MHBO is an independent net risk factor for stent occlusion, as reported in a study that evaluated 120 patients who underwent EUS-HGS.43
LIMITATIONS
Evidence of EUS-BD for MHBO remains scarce. Limited centers are capable of performing this technically demanding EUS intervention. The complexity of the procedures limits their use in day-to-day clinical practice.3 EUS-BD has a steep learning curve and requires approximately 40 procedures before gaining competence.44 However, comparison with other alternative modalities (e.g., PTBD) is scarce. Only available comparative data are available for CERES compared to PTBD.34
CONCLUSIONS
The advantages of EUS-BD over PTBD in MHBO with inaccessible papilla (Fig. 3DŌĆōF) include lower patient discomfort and internal drainage distant from the tumor, thus lowering the risk of tissue ingrowth and stent block. The non-communicating systems in MHBO can be drained with EUS-BD by placing a bridging stent between the right and left hepatic ducts (Fig. 4). EUS-guided interventions can be combined with ERCP, interventional radiology, and intraductal tumor ablation to treat complex MHBO. Stent occlusion post-EUS-BD can be managed with EUS or endoscopically guided interventions with plastic stents or covered SEMS in the majority without the need for rescue PTBD. Bile leaks and stent migration after EUS-BD can be minimized with proper techniques and choice of stents. Vascular injuries can be managed using transarterial therapies. The complexity of the procedure, steep learning curve, limited availability, and lack of comparative controlled studies with other modalities are current barriers to the widespread use of this technique.