CME for
KSGE members
Kosasih, Telisinghe, Lim, Metussin, Asli, and Chong: Chronic non-healing gastric ulcer in a patient with poorly controlled diabetes mellitus
Quiz
A 46-year-old man with non-replicative chronic hepatitis B infection and poorly controlled diabetes mellitus (glycosylated hemoglobin (HbA1c), 14.2%) with microalbuminuria, hypertension, and dyslipidemia, was referred for investigation of his chronic dyspepsia. Upper gastrointestinal endoscopy performed at another center showed a large excavating ulcer at the incisura (measuring 4 cm) with a firm necrotic base. Biopsies from the margin of the ulcer were negative for malignancy but positive for chronic gastritis, intestinal metaplasia, and Helicobacter pylori. It also showed Candida spores and possible hyphae elements. Repeat evaluation was recommended. The patient was administered H. pylori eradication therapy with 10 days of omeprazole 20 mg twice daily, amoxicillin 1 g twice daily, and clarithromycin 500 mg twice daily followed by maintenance omeprazole (20 mg twice daily). Due to the concern for underlying malignancy, a computed tomography (CT) scan was performed and showed a thickened lesser curve (thickest, 2.5 cm) with peri-gastric, celiac, and retroperitoneal lymphadenopathies ( Fig. 1). The largest lymph node measured 1.5 cm. The remaining organs were normal. A repeat endoscopy two months after H. pylori eradication therapy showed a non-healing of the ulcer with a less necrotic base ( Fig. 2). It was not possible to properly visualize the ulcer base because of its location and depth. Biopsies were obtained from the ulcerтs margins and base. Results from the biopsies were again negative for malignancy, but this time the diagnosis was confirmed ( Fig. 3). Furthermore, staining for H. pylori returned negative results. What is the most likely diagnosis?
Answer 
Repeat biopsies from the ulcer base showed irregular branching non-septate thick hyphae of mucormycosis ( Fig. 3), which was more obvious with Grocott methenamine silver staining, leading to the diagnosis of primary gastric mucormycosis. The patient was treated with a prolonged course (18 weeks) of antifungal therapy (four weeks of intravenous liposomal amphotericin, followed by oral posaconazole). After 28 days of liposomal amphotericin treatment, the patientтs endoscopy revealed significant shrinkage of the gastric ulcer, and biopsies were negative for fungal hyphae. Prolonged antifungal treatment was required due to interruption in the treatment schedule because of acute kidney injury and severe electrolyte imbalance from the antifungal treatment. Endoscopy six months after diagnosis showed complete healing of the ulcer. Mucormycosis is an opportunistic angio-invasive fungal infection that affects patients who are immunocompromised, either from their disease or treatment. 1-3 However, some patients may not have any apparent risk factors. 2 Acquisition from the environment is through inhalation or direct inoculation of the fungal spores (sporangiospores). 1 Among the varied manifestations, rhino-cerebral and pulmonary forms are the most common, while primary gastrointestinal mucormycosis is relatively rare. 1-3 Stomach is the most commonly affected part of the gastrointestinal tract followed by the colon and the ileum. Involvement can be either isolated or part of systemic involvement. In gastrointestinal mucormycosis, acquisition may occur through ingestion of infected sputum or secondary colonization of pre-existing ulcers. A literature review of publications between 2015 and 2021 identified 87 cases of gastrointestinal mucormycosis. A review of 80 cases (70 adults and 10 neonates) with available data showed that Asia has the highest incidence (57.5%), 3 with gastric mucormycosis being the most common. Corticosteroids and poorly controlled diabetes mellitus were the two most common risk factors. Abdominal pain, fever, and visceral perforations were the most common manifestations. 3 The condition can also present with emphysematous gastritis and fistula. 4,5 In almost all cases (97%), diagnoses were made through histopathological examinations, whereas culture and molecular methods were used in 28% and 17%, respectively. 3 Surgery with antifungal therapy was used in 61% of reported cases, followed by antifungal monotherapy in 28%, and surgery alone in 11%. 3
In our case, the diagnosis was delayed because the first biopsies were not conclusive. Most of the disease activity was submucosal, as shown by the CT scan, which may explain the discrepancy between the first biopsies taken from the margin of the ulcer and the second biopsies taken from both the margin and the base of the ulcer. This may also explain the low density of fungal hyphae present ( Fig. 3). Histological examination with appropriate Grocott methenamine silver staining is important to detect cases where fungal elements are not florid. In our case, mucormycosis colonization was an unlikely diagnosis for several reasons. Histology showed necrotic changes between fungal hyphae elements typically seen in invasive mucormycosis. More importantly, the ulcer only started to regress and heal after the initiation of antifungal therapy. Diffuse thickening of the gastric lesser curve seen on CT also indicated diffuse submucosal involvement from the infection. Ideally, patients should maintain good diabetes control to prevent the recurrence of mucormycosis. In our case, the diabetes control improved during and at the end of treatment with a reduction of serum HbA1c to 8.7%. However, this improvement was not maintained, with the latest serum HbA1c measuring at 11%, four years after recovery from mucormycosis. To date, the patient has not experienced a recurrence of the dyspepsia or developed any other symptoms suggestive of the recurrence of mucormycosis. In conclusion, this case highlights that mucormycosis should be considered in patients with chronic non-healing ulcers after common etiologies, such as underlying malignancy, have been excluded, especially if risk factors, such as poorly controlled diabetes mellitus, are present.
Fig.Т 1.
Axial (A) and coronal (B) contrast-enhanced computed tomography images show diffuse thickening of the stomach wall, mainly of the submucosal layer along the lesser curvature (arrowheads), with reactive peri-gastric adenopathy (arrows). 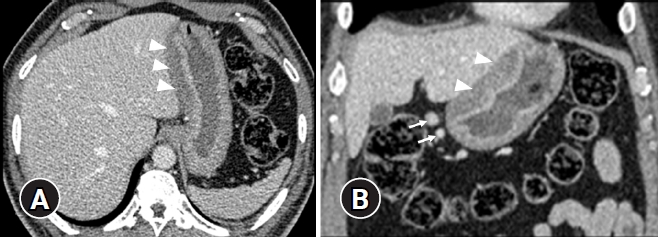
Fig.Т 2.
Endoscopic findings. An incisura showing edematous mucosa with a deep penetrating ulcer with narrowing of the gastric lumen (A), and mild erosions in the otherwise normal antrum (B). 
Fig.Т 3.
(A) Hematoxylin and eosin stain of gastric biopsy showing fungal hyphae amongst necrotic tissues (У200). (B) Grocott methenamine silver stain showing the fungal hyphae more clearly (У200). 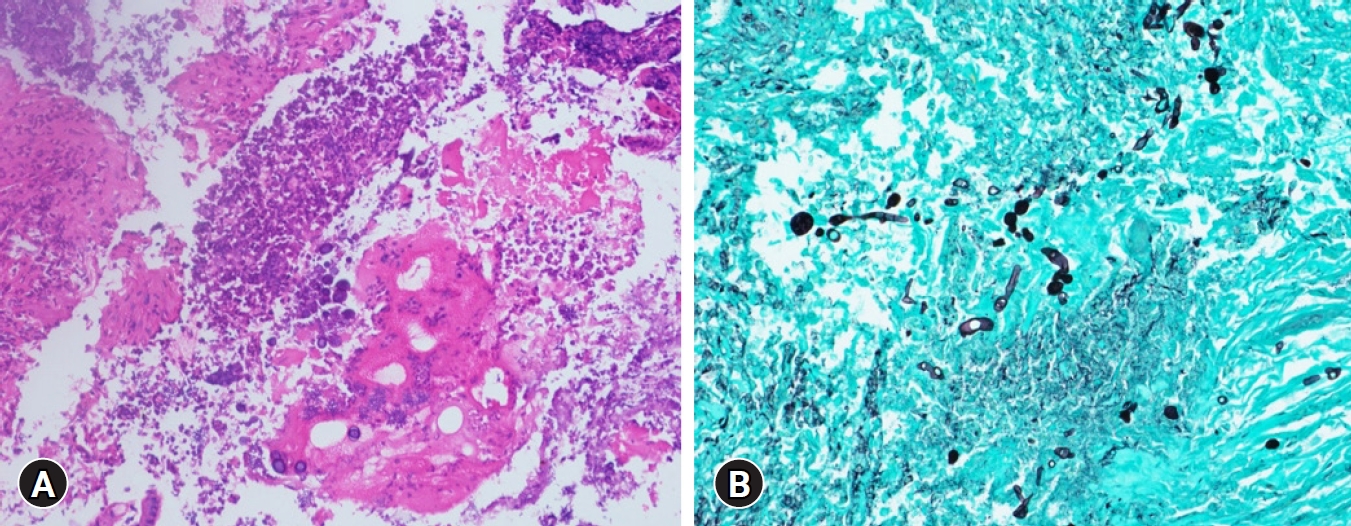
REFERENCES
1. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634т653.   2. Patel A, Kaur H, Xess I, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect 22020;26:944т950.  3. Didehdar M, Chegini Z, Moradabadi A, et al. Gastrointestinal mucormycosis: a periodic systematic review of case reports from 2015 to 2021. Microb Pathog 2022;163:105388.  
|
|

