Role of radiofrequency ablation in advanced malignant hilar biliary obstruction
Article information
Abstract
Malignant hilar biliary obstruction (MHO), an aggressive perihilar biliary obstruction caused by cholangiocarcinoma, gallbladder cancer, or other metastatic malignancies, has a poor prognosis. Surgical resection is the only curative treatment for biliary malignancies. However, the majority of patients with MHO cannot undergo surgery on presentation because of an advanced inoperable state or a poor performance state due to old age or comorbid diseases. Therefore, palliative biliary drainage is mandatory to improve symptomatic jaundice and the quality of life. Among the drainage methods, endoscopic biliary drainage is the current standard for palliation of unresectable advanced MHO. In addition, combined with endoscopic drainage, additional local ablation therapies, such as photodynamic therapy or radiofrequency ablation (RFA), have been introduced to prolong stent patency and survival. Currently, RFA is commonly used as palliative therapy, even for advanced MHO. This literature review summarizes recent studies on RFA for advanced MHO.
INTRODUCTION
Cholangiocarcinoma, gallbladder cancer, hepatocellular carcinoma, lymphoma, and other metastatic tumors can result in malignant perihilar biliary blockage. The most prevalent predisposing condition among them is hilar cholangiocarcinoma, which accounts for >50% of all cholangiocarcinomas.1-5 Although surgical resection remains the primary line of treatment for advanced malignant hilar biliary obstruction (MHO), most patients have poor prognosis because of the advanced stage at which they are initially discovered. Therefore, regardless of whether surgical resection is performed, primary biliary decompression is essential until the end of life. Despite debates on the strategy of biliary drainage, multiple drainages for >50% of the liver volume are mandatory. Additionally, if experienced endoscopists are available, endoscopic drainage is considered a primary palliative method.
Various treatment options are available following palliative decompression for unresectable MHO, such as traditional chemotherapy, immunotherapy, and locoregional therapies such as transarterial chemoembolization, external beam radiation therapy, photodynamic therapy (PDT), and radiofrequency ablation (RFA).6 Among these, endoscopic intraductal local ablation therapies such as PDT or RFA may be potential endoscopic treatments to increase stent patency and survival.7-11 Theoretically, local therapy can induce prolongation of stent patency, which may reduce stent-related adverse events and have a survival benefit. Numerous studies have reported the benefits of PDT and RFA.7-11
The application of intraductal RFA (ID-RFA) to obstructive tumors within the common bile duct before stent placement is thought to have the potential to postpone the growth of the tumor and, consequently, the recurrence of biliary blockage. Compared with PDT, RFA does not require a photosensitizer and is easy to perform endoscopically. This in-depth review aimed to understand the current role and efficacy of RFA in the palliative treatment of MHO.
RECENT UPDATE OF ENDOSCOPIC PALLIATION
The Asia-Pacific consensus and the European Society of Gastrointestinal Endoscopy guidelines suggest percutaneous biliary drainage or combination with an endoscopic method rather than primary endoscopic drainage for bilateral or multi-segment drainage in advanced MHOs, such as Bismuth type II or higher. This recommendation was based on the liver volume drainage of >50% without increasing the rate of adverse events.5,12 According to the factor analysis used to forecast drainage efficacy following endoscopic stenting for MHO, biliary drainage >50% is linked to a longer survival time than drainage <50% (119 days vs. 59 days, p=0.005).13 Draining >50% of the liver volume is a major factor in drainage effectiveness, particularly for high-grade strictures such as Bismuth type III or IV.13
Previously, percutaneous drainage was preferred in advanced MHO because of selective lobar selection and the relatively lower technical success rate of endoscopic drainage. However, the choice of percutaneous or endoscopic drainage should be made based on the environment, including the features of the disease, experts, and hospitals. Each of these two approaches has its benefits and drawbacks. The favored method has recently been the endoscopic method, but it is complementary rather than competitive. According to the 2021 American Society of Gastrointestinal Endoscopy (ASGE) guideline,14 for patients with unresectable MHO, the ultimate decision regarding the palliative draining strategy should be based on the patient’s preferences, disease characteristics, and local expertise.
In recent years, in addition to endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic biliary drainage (PTBD), endoscopic ultrasound-guided biliary drainage (EUS-BD) has become popular worldwide as an alternative when ERCP is impossible or fails and for re-intervention for recurrent biliary obstruction.15 Nakai et al.16 performed EUS-BD in 88 patients with MHO and reported a technical success rate of 98%, a clinical success rate of 77%, and a complication rate of 8%. A multicenter comparison of the usefulness of ERCP and EUS-BD combined with PTBD as a drainage method for MHO showed that ERCP and EUS combined had a significantly lower rate of recurrent biliary obstruction than PTBD.17 On the contrary, the EUS-BD is not yet performed in all facilities because of insufficient device development, and its radiation exposure is still higher than that of ERCP.18 Depending on future device development, EUS-BD has the potential to become a more viable option for MHO.
Regarding stent components, a plastic stent is simple to replace, remove, and add for more efficient drainage. It does not hinder other treatments such as local ablation therapies or surgery. Therefore, plastic stents are recommended for preoperative biliary drainage. In addition, stent size can be adjusted to the common bile duct diameter, which is not dilated in most MHO cases. However, a considerably greater rate of stent malfunction occurs because of the smaller diameter, necessitating frequent stent replacement during the survival period, which may reduce quality of life and increase expenses.19
Metal stents with comparatively larger diameters than plastic stents offer longer stent patency than plastic stents. The side branches of the intrahepatic ducts or cystic duct are not blocked by an open wire mesh metal stent. Technically, because longer plastic stents are prone to kink in severe or constrictive strictures, the insertion by a pushing catheter may be challenging. However, a self-expandable metal stent (SEMS) is preloaded in a delivery catheter via a narrow delivery system (5.4 to 8.5 Fr, per the manufacturer), which makes it easier to pass through constricted biliary strictures and improves pushability.20-23 Clinical studies comparing SEMS with plastic stents in MHO have shown that SEMS has a higher rate of technical and clinical success with prolongation of stent patency by reducing the number of reinterventions, leading to its cost-effectiveness.19,24-27 SEMSs have been employed primarily for MHO palliation. Metal stents have been recommended for patients with high-grade MHO, with a predicted survival of >3 months in both the 2013 Asia-Pacific consensus and the 2018 European Society of Gastrointestinal Endoscopy guidelines. For palliation, SEMS outperformed plastic stents in terms of clinical outcomes and cost-effectiveness.5,28 However, the current drainage strategy has changed. In patients with a limited life expectancy (3 months) and those who placed high importance on avoiding repeated interventions, a recent ASGE guideline from 2021 supports metal stents rather than plastic stents. However, mandatory SEMS insertion should be avoided if the future treatment strategy is inconclusive.14
RFA IN MHO
Mechanics of ID-RFA
RFA has been introduced and used as a local treatment method for various malignant tumors such as liver and kidney malignancies. After an electrode is inserted into the target lesion under ultrasound or computed tomography guidance, a high-frequency alternating current is applied to induce ionic agitation in cancer cells. This treatment results in coagulation necrosis at the tumor site, as intracellular and extracellular water is evaporated by frictional heat within the tissue.29
The frictional heat produced during this period is inversely proportional to the distance from the electrode and is related to the voltage and duration of the high-frequency current. If the heat generated by RFA is ≥50°C, irreversible cell damage occurs because of cell wall destruction and protein denaturation; thus, a therapeutic effect can be expected. However, if it is kept at ≥100°C, coagulum develops in the tissue surrounding the RFA catheter tip, increasing the current’s resistance and lowering the effectiveness of RFA. Therefore, a method for maintaining an appropriate temperature by positioning a temperature sensor in the RFA catheter was used.
In general, RFA devices used for other solid cancers are mainly monopolar devices that attach a grounding pad to other parts of the body and an RFA catheter is placed at the target lesion site. However, in RFA in the bile duct, a bipolar method is used, in which an even number of electrodes are attached to the catheter to induce cell necrosis using high-frequency waves generated from both electrodes.
RFA devices
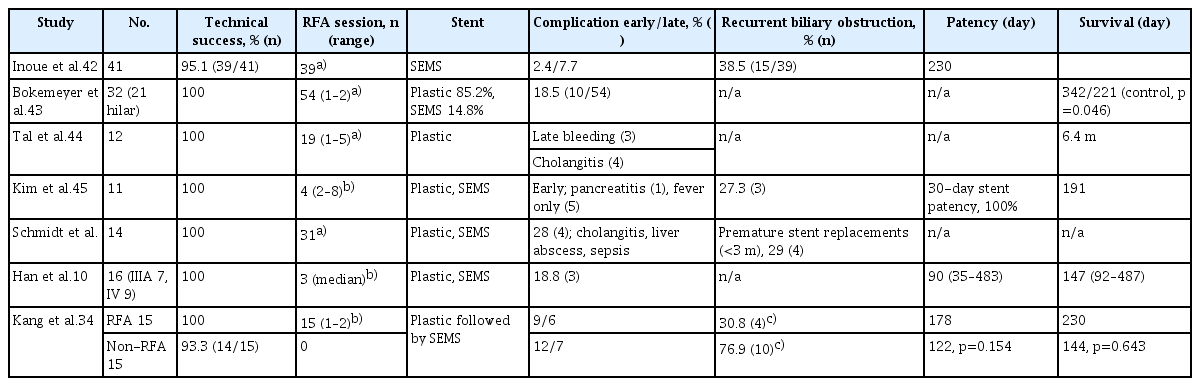
The Habib HPB-RF probe (Boston Scientific, Natick, MA, USA) and Endo Luminal Radiofrequency Ablation (ELRA) RF catheter (STARmed, Goyang, Korea) are currently available for use in intrabiliary RFA (Fig. 1). Over a 0.035-inch guidewire, a 1.8-m long, 8-Fr bipolar RFA catheter (Habib EndoHPB; EMcision Ltd., London, UK) is introduced into the biliary tree. Endoscopes with a functional channel of at least ≥3.2 mm can be used. The distal end of the RFA catheter features a 5-mm leading tip, and its proximal end contains two circumferential 8 mm-wide stainless-steel electrodes that are spaced 8 mm apart to provide 25 mm of effective cylindrical ablation. The catheter’s proximal end is connected to an electrosurgical generator, and the generator settings should be chosen in accordance with the manufacturer’s instructions or through ex vivo experiments on animal tissue.30

Device of intraductal radiofrequency ablation (ID-RFA). (A) Habib EndoHPB catheter (EMcision Ltd., London, UK). The use of an adapter cable enables bipolar RFA and prevents the need for electrode grounding pads. It has two 8-mm long electrodes, compatible with commonly available RF generators, and endoscopes with a working channel of ≥3.2 mm. The usable total length is 180 cm and 8 Fr (2.7 mm) in diameter. (B) Endo Luminal Radiofrequency Ablation (ELRA) RF catheter (STARmed, Goyang, Korea) and VIVA Combo generator (VCS10; STARmed). The exposed tip length of the catheter is 11, 18, 22, and 33 mm in size according to the anatomy or length of the stricture (left). For example, in the 33-mm electrode, after a 9-mm leading tip and 7-mm insulated portion, four 6-mm electrodes are separated by 3-mm insulated segments. The catheter diameter is 7 Fr, and the total length is 175 cm (center). The temperature-sensing system of ensuring consistent temperature in the ablation area with VIVA Combo generator’s impedance monitoring system prevents overapplication of energy (right).
The ELRA RFA probe is an endoscopically deployed 7-Fr (2.3 mm) bipolar device with a working length of 175 cm. In addition to carrying a monitoring function with feedback to the specific VIVA COMBO generator (STARmed), the distal end of the ELRA catheter features a 2-ring or 4-ring application that serves as the main component of the ablation device. A defined energy application of 7 to 10 W for 1 to 2 minutes can be set up to produce a consistent thermal ablative effect across the distal and proximal electrode borders along the entire length.29,31
Preclinical data
Although the clinical efficacy of ID-RFA has been previously demonstrated in human clinical studies, several concerns remain regarding potential ID-RFA-related morbidity and mortality. It is unclear how safe ID-RFA is. Animal studies, both ex vivo and in vivo, have been performed to assess the safety and efficacy of RFA.
First, regarding the Habib RFA device, Itoi et al.32 tested the RFA probe in energy settings of 5, 10, 15, and 20 W at durations of 60, 90, and 120 seconds using freshly harvested pig livers. Even though this was an ex vivo animal study, it proposed that ablation at 7 to 10 W of power for two minutes may be suitable for RFA using a Habib-EndoHBP catheter based on each setting. Zacharoulis et al.33 also performed an animal study using a Habib probe. They tested the energy settings of 1 to 10 W in porcine bile ducts and sacrificed pigs 24 hours later. The bile duct wall had superficial-to-intermediate depth of damage at levels between 2 and 5 W. The normal porcine bile duct underwent full-thickness coagulation at levels of 6 to 7 W for 50 to 60 seconds, frequently with ductal perforation. The nearby duodenum and pancreas suffered thermal damage as a result of the application of 8 to 10 W.
Second, using a temperature-controlled ID-RFA, ELRA probe, Cho et al.29 proposed that the viable and efficient setting for ID-RFA using an ELRA RF catheter in mini-pigs was target temperature (75°C–80°C)-based RFA ablation using a power of 7 to 10 W for 120 seconds. In comparison to EB-RFA using an 18-mm 7-W electrode, the microscopic maximum damage depth and ablation area of EB-RFA using a 33-mm 10-W RFA electrode were substantially deeper and larger (median: 2.7 vs. 2.1 mm, p=0.004; 48.9 vs. 36.2 mm2, p=0.016). However, the microscopic ablation parameters did not differ significantly between the two RFA target temperatures (75°C vs. 80°C).
However, these ex vivo and in vivo studies were performed in the normal bile ducts of mini-pigs, and it is difficult to represent tumorous conditions, especially perihilar malignancies. MHO also differs from the extrahepatic bile duct. Using preclinical data of an experimental mini-pig study, Kang et al.34 performed temperature-controlled ID-RFA in the perihilar lesion. Moreover, Cho et al.29 set the ID-RFA mode at 7 W, 80°C, and adapted different times: 60, 90, and 120 seconds.
Therefore, based on these animal studies, ID-RFA may have a safe and effective ablative effect on tumor lesions without increasing adverse events. However, larger controlled trials are required according to the set-up mode and position of the tumor.
Endoscopic procedure of ID-RFA
To determine the length, diameter, and location of the stricture, the bile duct was first cannulated. The RF catheter was then advanced across the guidewire once the radiopaque electrodes were positioned at the perihilar stricture, following the insertion of guidewires into both intrahepatic ducts. The stricture length can be used to determine the electrode length of an RF catheter. Additionally, overlapping RFA is performed while moving from the distal to the proximal stricture margin. Serial overlapping ablations can completely ablate malignant strictures. The RFA probe was then used with the selected energy, temperature, and duration settings (Fig. 2). In contrast to extrahepatic cholangiocarcinoma, advanced MHO requires further studies with acceptable power, duration, and method. In a curved or tortuous hilar area, there are cases where it is difficult for the RFA catheter to effectively contact all areas of the lesion compared to the extrahepatic bile duct. After the completion of ablation therapy, any remaining coagulated tissue debris can be removed using balloon sweeps, and a cholangiogram can be used to detect biliary complications, such as perforation. However, balloon cleaning is not always needed. A metal or plastic biliary stent is implanted to ensure bile drainage. Bilateral plastic stents are advised if repeated RFA is planned. When RFA is used to treat an obstructed SEMS, other operations such as balloon dilation and insertion of more stents could be performed. Prophylactic antibiotic can reduce the risk of infection.30,34,35
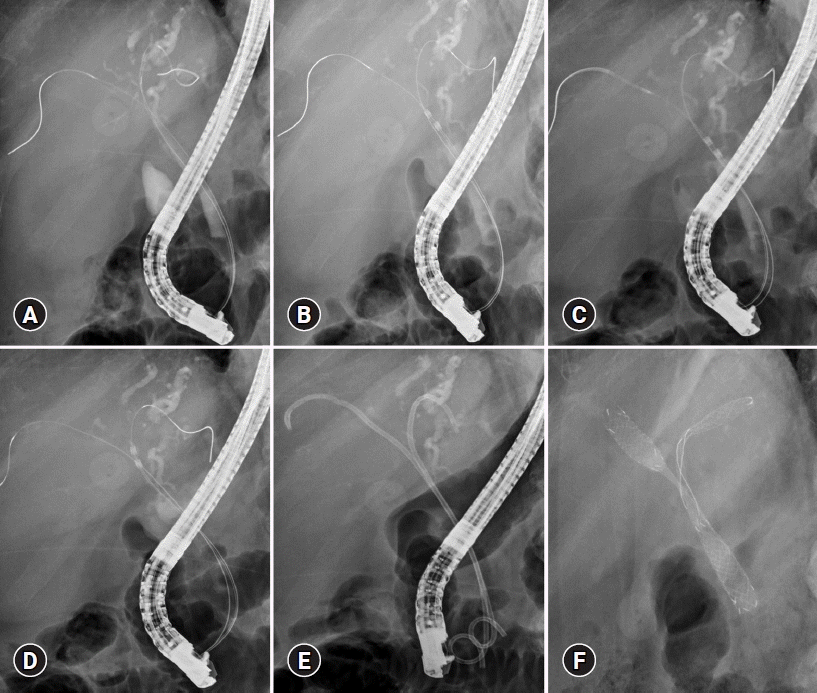
Intraductal radiofrequency ablation in advanced malignant hilar biliary obstruction. After placing guidewires bilaterally (A), the radiofrequency ablation catheter (Endo Luminal Radiofrequency Ablation [ELRA] RF catheter, STARmed; 11 mm probe; 80°, 7 W, 120 seconds) was advanced to both intrahepatic ducts over the guidewire sequentially. Then, bilateral strictures were ablated (B-D). Finally, plastic stents were inserted bilaterally, then exchanged with uncovered self-expandable metal stent bilaterally after 3 months (E, F).
Clinical outcomes of ID-RFA
Ortner et al.36 and Zoepf et al.37 found that PDT with stents prolonged the survival and enhanced Karnowski performance status compared with biliary stenting alone in patients with unresectable cholangiocarcinoma. Patients receiving PDT for hilar cholangiocarcinoma also showed a trend toward prolonged biliary stent patency times (compared with those receiving stents alone).38 Moreover, previous studies have reported that PDT may be beneficial for patients with hilar cholangiocarcinoma.38,39
According to a recent systematic review and meta-analysis of 55 studies, PDT is more effective than RFA or stenting in the treatment of unresectable extrahepatic cholangiocarcinoma.40 Using PDT, RFA, and stenting alone, the pooled overall survival rates were 11.9 months (95% confidence interval [CI], 10.7–13.1), 8.1 months (95% CI, 6.4–9.9), and 6.7 months (95% CI, 4.9–8.4), respectively. Additionally, the pooled survival rates for patients who underwent endoscopic and percutaneous RFA were 12 months (95% CI, 9.8–14.3) and 5 months (95% CI, 3–6.9), respectively. The pooled 30-day mortality rate among patients undergoing PDT was 3.3% (95% CI, 1.6%–6.7%) compared with a rate of 7% (95% CI, 4.1%–11.7%) among those undergoing RFA. PDT appeared to demonstrate better results than the RFA or simple stent implantation.40 These investigations did not focus solely on high-grade MHO, but they did raise the possibility that PDT could be more efficient as a local tumor treatment. However, large-sized randomized controlled trials (RCTs) are constrained, and PDT has several drawbacks, including the potential for photosensitivity and need for expensive, time-consuming procedures and highly specialized equipment. Furthermore, it is unclear whether chemotherapy, radiation therapy, local ablation therapy, or a combination of these treatments leads to better results.
Similar to PDT, RFA is a unique form of local ablation. It does not require a phototoxic photosensitizer. In addition, the endoscopic technique for ERCP is relatively straightforward. RFA is now commonly employed because of its accessibility, despite insufficient data to prove its parity with or superiority to PDT in MHO. Its outcomes are comparable with those of PDT. Therefore, for palliative treatment of malignant biliary obstruction, RFA may be a potent therapeutic option for PDT (Table 1).10,34,41-45
However, there are limited data on well-designed RCTs in advanced MHO. In low-grade obstructions such as Bismuth type I or II, which are inoperable or difficult to perform, the RFA and stenting group showed longer stent patency and survival than the stent-only group.8 Still, the effect for high-grade advanced MHO such as Bismuth types III and IV needs further research. In addition, the safe and effective control mode of the RFA probe for advanced MHO remains unclear. In contrast to distal biliary obstructions, perihilar malignant obstructions are relatively difficult to treat. In addition, the effective length and setting mode vary according to the stricture length and anatomy level. Therefore, more large-scale RCTs should be performed to determine the most effective and safe mode of therapy for high-grade MHO.
Regarding adverse events, unlike PDT, patients often complain of pain during the ID-RFA procedure. In addition, previous investigations have noted cholangitis, hepatic abscesses, and mortality from acute bleeding (hemobilia) during RFA in MHO.41-45
A recent preliminary RCT by Kang et al.34 revealed the efficacy of temperature-controlled ID-RFA for advanced MHO. In this preliminary study, no differences were found in stent patency (178 days in the RFA group vs. 122 days in the non-RFA group, p=0.154) and overall survival (230 days vs. 144 days, p=0.643). However, with each stricture length more than 11 mm on both sides, the stent patency in the RFA group was longer than that in the non-RFA group (175 days vs. 121 days, p=0.028). Patients who received RFA had a higher chance to exchange stents with metal stents regularly without early malfunction of the initial plastic stents (69.2% vs. 23.1%, p=0.018). Adverse event rates also did not differ between the stent-only groups. Although this preliminary study enrolled a small number of patients, it proved the benefit and safety of RFA in advanced high-grade MHO based on a bench test of the porcine model.
Another concern of RFA is the set of power modes. Because the wall of the perihilar duct is thinner and has a more complex duct-vascular contact than the extrahepatic duct, the risk of RFA-induced complications may be severe. Power controlled ID-RFA has reported that serious complication such as hemobilia can be occurred. However, temperature-controlled RFA systems may reduce serious complications. Studies using temperature-controlled RFA have reported no serious adverse events10,34,45; however, more large-sized studies, including RCTs are required.
According to the 2021 ASGE guidelines, topical treatments such as RFA and PDT delivered through SEMS can be used at research institutions, tertiary referral hospitals, and higher levels of care.14 Therefore, simultaneous administration of local endoscopic ablation therapies and active chemotherapy is anticipated to increase stent patency, reduce complications, and improve survival rates compared with stenting alone.
CONCLUSIONS
The ultimate goal of endoscopic palliation for inoperable or inappropriate MHO is to prolong stent patency and survival, which may also improve the quality of life by preventing the need for further intervention or negative outcomes. To provide effective endoscopic palliation with or without local ablative therapies, chemotherapy, or radiation therapy, a more important consideration is the patient’s physical status and disease characteristics. To minimize the tumor burden of MHO and/or to increase stent patency and/or survival, RFA can be conveniently performed as a local ablation therapy during ERCP. However, still, little data are available on advanced MHO. To confirm the effectiveness and safety of advanced MHO, further large-scale research is required.
Notes
Conflicts of Interest
Mamoru Takenaka and Tae Hoon Lee are currently serving as an associate editor and a section editor of Clinical Endoscopy, respectively; however, they had not involved in the peer reviewer selection, evaluation, or decision process of this article. The authors have no potential conflicts of interest.
Funding
This study was supported by the Soonchunhyang University Research Fund.