Exploring quality indicators for the detection of Helicobacter pylori-naïve gastric cancer: a cross-sectional nationwide survey
Article information
Abstract
Background/Aims
Diagnosis of Helicobacter pylori-naïve gastric cancer (HPNGC) is becoming increasingly important. This study aimed to explore the quality indicators for HPNGC detection.
Methods
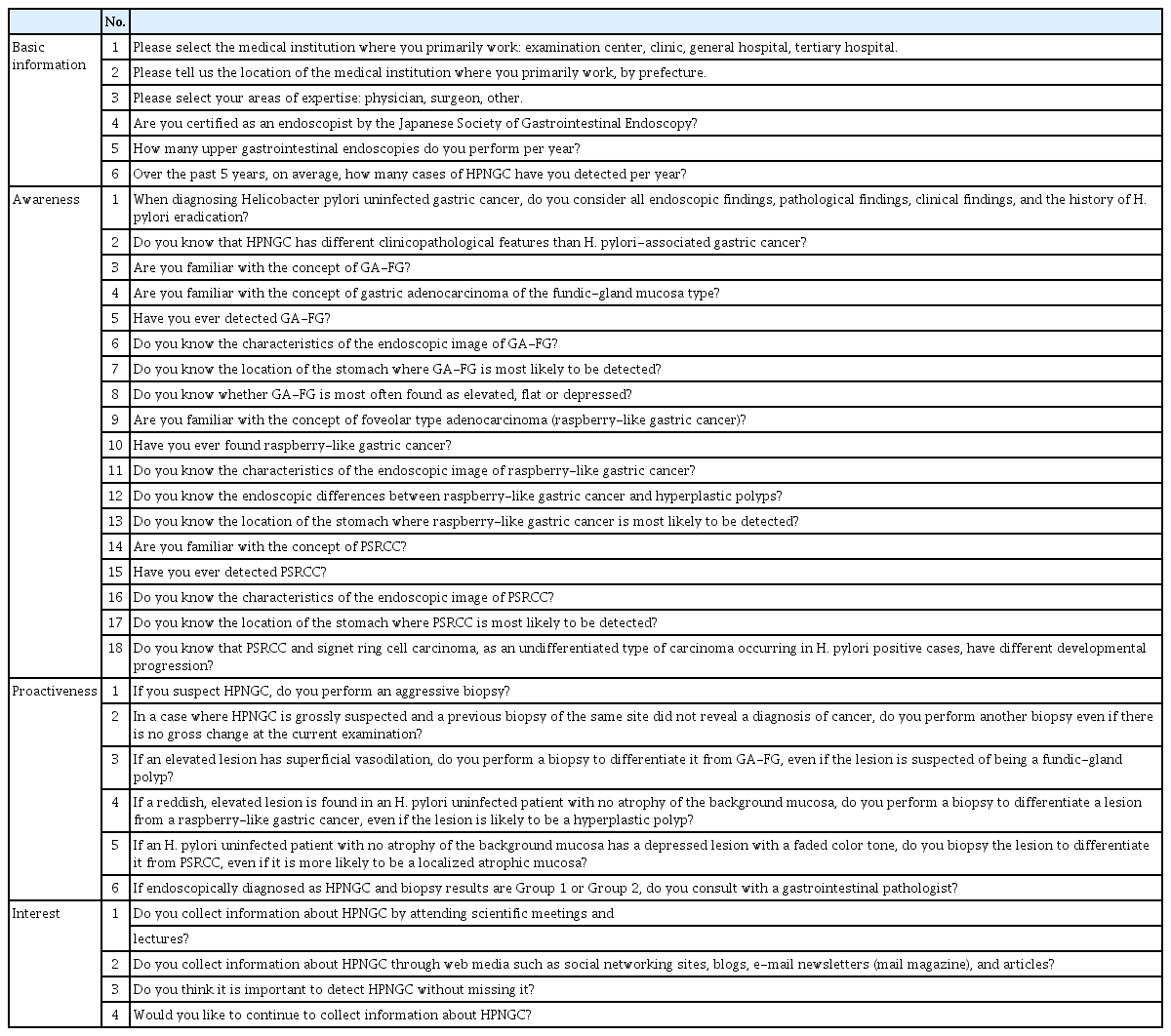
We conducted a cross-sectional, nationwide, web-based survey of gastrointestinal endoscopists in Japan. In addition to questions about the number of HPNGC cases detected in a year and basic information, the questionnaire also consisted of 28 questions: (1) 18 about HPNGC awareness, (2) six about diagnostic proactiveness, and (3) four about interest in HPNGC.
Results
Valid responses were obtained from 712 endoscopists. The Japan Gastroenterological Endoscopy Society-certified endoscopists had a significantly higher HPNGC detection rate than the nonspecialists (0.42% vs. 0.32%, respectively; p=0.008). The results of the multiple regression analysis showed that Japan Gastroenterological Endoscopy Society certification and high awareness and interest scores were independent predictors of the HPNGC detection rate (p=0.012, p<0.001, p=0.024, respectively). Principal component analysis showed that the endoscopists who attended conferences for collecting information on HPNGC had a higher level of awareness.
Conclusions
To improve the detection of HPNGC, it is necessary to increase the awareness of the disease. It is hoped that relevant societies will play an important role in endoscopists’ education.
INTRODUCTION
Helicobacter pylori (HP) infection plays an important role in the development of gastric cancer.1,2 Several randomized control trials have shown that HP eradication reduces the risk of carcinogenesis.3,4 The widespread use of HP eradication therapy has reduced the incidence of new gastric cancer cases5 and decreased the prevalence of HP in Japan over time.6,7
HP-naïve gastric cancer (HPNGC), rather than HP-associated gastric cancer, has been increasingly detected in recent years. The first reported HPNGC was pure signet ring cell carcinoma (PSRCC).8 With the increased awareness of PSRCC, it has become a major component of reported HPNGC cases.9 Studies on the genomic landscape of PSRCC have shown that not all PSRCCs carry the CDH-1 mutation, making it a hereditary and a sporadic cancer.10,11 The second reported HPNGC was gastric adenocarcinoma of the fundic-gland type (GA-FG).12-14 GA-FG was initially reported as HPNGC, but later shown to also occur in HP-positive or post-HP eradication mucosa, making it important to understand endoscopic findings.15 Raspberry-like tumors are treated as adenocarcinomas in the Japan Gastric Cancer Association guidelines16 but are internationally reported as foveolar-type gastric adenoma.17
The Lauren and World Health Organization (WHO) classifications are often used to classify gastric cancer.18-20 HPNGC, such as PSRCC, GA-FG, and raspberry-like tumors, have different endoscopic findings compared to the intestinal-type gastric cancer, including tubular adenocarcinoma, according to the Lauren classification. Localized atrophy, submucosal tumors, and hyperplastic polyps are similar in appearance and may not be diagnosed by biopsy without an accurate understanding of their characteristics.21 Experience with endoscopy is necessary to differentiate HPNGC from these common, benign changes. Indeed, we have reported the importance of the number of years of endoscopic experience and gastric observation time as quality indicators for HPNGC detection.22 We have also reported that for the efficient HPNGC detection, a review by another endoscopist is effective in controlling endoscopic quality.23 However, HPNGC does not have a widely recognized and consensus classification worldwide, and its disease concept is still being established.19,20 The WHO classification and other criteria for the classification of gastric cancers define them as tumors that invade the submucosa; therefore, HPNGC, such as raspberry-like tumors with a low rate of invasion into the submucosa, have been classified as adenomas or dysplasias. Differences in the diagnostic criteria for HPNGC in Japan and other countries may hinder the understanding of this disease.24 The only way for endoscopists to expand their understanding of HPNGC is to actively collect information by attending scientific meetings and educational lectures, or by searching the literature; therefore, disease awareness is an urgent issue. Furthermore, real-world data on the endoscopists’ actual understanding of HPNGC in Japan and the number of cases diagnosed annually are lacking. The purpose of this study was to determine the endoscopists’ awareness and interest in HPNGC in their practice through a nationwide survey in Japan and to explore the quality indicators for HPNGC detection.
METHODS
Study design
This was a cross-sectional study of endoscopists in Japan using a web-based questionnaire survey.
Survey participants
Endoscopists from all over Japan were included in the study. Subscribers to the Endoscopy Atlas, an e-mail newsletter published by Toshiaki Hirasawa for endoscopists in Japan and endoscopists at facilities affiliated with the authors and research collaborators were eligible. On November 1, 2021, an e-mail was sent to all participants simultaneously, explaining the study outline. If they agreed to participate, they were directed to a system that allowed them to respond to a web-based questionnaire with anonymity assured. The deadline for obtaining consent and response was November 15, 2021.
The study participants who gave consent were asked about the type of facility they worked in (health checkup facility [examination center], clinic, general hospital, or tertiary hospital), the prefecture where their facility was located, their specialty (physician, surgeon, or other), whether they were certified by the Japan Society of Gastroenterological Endoscopy (JGES), and the number of endoscopies they performed in a year. The basic attributes of the survey participants were also analyzed.
Questionnaire list
HPNGC is defined as gastric cancer in which HP infection is not involved in carcinogenesis. Specifically, HPNGC includes PSRCC, GA-FG, and raspberry-type tumors. At the beginning of this survey, the study participants were informed that HPNGC was defined as gastric cancer found in patients who were not infected with H. pylori. “HP naïve” status was determined by at least two of the following tests: serum anti-HP-IgG antibody, urea breath test, HP antigen in stool, and direct observation of HP in biopsy specimens; additionally, the absence of gastric atrophy and prior HP eradication treatment were mandatory. In addition to questions about the actual number of HPNGC cases detected in a year, the questionnaire also included a total of 28 questions as follows: (1) eighteen were about their awareness of HPNGC (two general questions, six about GA-FG, five about raspberry-like tumors, and five about PSRCC); (2) six about their diagnostic proactiveness (e.g., frequency of biopsy and attitude towards consulting a gastrointestinal pathologist); and (3) four on their interest in the disease (e.g., whether the endoscopist attended conferences and educational lectures and whether the endoscopist used web media such as article searches, social network services, e-mail newsletters, and blog articles) (Table 1). All questions were answered with “yes” or “no”.
Data analysis
The scores for awareness, diagnostic proactiveness, and interest in the disease were based on the number of "yes" answers given to each question out of all questions (out of 100 points). In addition, for questions about awareness, the answers for GA-FG, raspberry-like tumors, and PSRCC were scored separately on a 100-point scale based on the percentage of “yes” answers to each question.
In the first step of the analysis, experience in diagnosing HPNGC was used as the primary outcome to determine its relationship with endoscopist characteristics. We first investigated whether there were differences in the experience in diagnosing HPNGC based on the endoscopists’ background. We then investigated whether there were differences in awareness, diagnostic proactiveness, and interest in the disease based on the endoscopists’ background.
The second step of the analysis was to build a predictive model for the HPNGC detection rate. Candidate predictors were explored using logistic regression analysis, with the endoscopists’ background factors, awareness, diagnostic proactiveness, and interest in the disease as the explanatory variables and experience in diagnosing HPNGC as the objective variable. The odds ratio (OR) and 95% confidence interval (CI) for each variable in this model were used to identify the candidate predictors for HPNGC detection.
In the last step of the analysis, principal component analysis (PCA) was performed based on all the parameters of all the endoscopists to determine the candidate predictors of HPNGC detection extracted in the second step of the analysis.
All statistical analyses were performed using the R software 4.0.4 (The R Foundation for Statistical Computing, Vienna, Austria).25 Student t-test was used to compare the means between the two groups, one-way ANOVA was used to compare three or more means, and multiple comparisons were made between all the groups using the Bonferroni method. In the logistic regression analysis, the OR with 95% CI and the p-value of each explanatory variable compared with the reference were calculated. PCA was performed using the prcomp package of R software. The ggbiplot package of R and PRISM software were used to draw the graphs. The mean values in the text and graphs are presented as mean±standard deviation. Statistical significance was set at p-value <0.05.
Ethical statements
This study was approved by the Institutional Review Board of the Shinjuku Tsurukame Clinic on October 19, 2021 (approval number: 2105) and was conducted from November 1 to November 15, 2021.
RESULTS
Baseline characteristics of the study participants
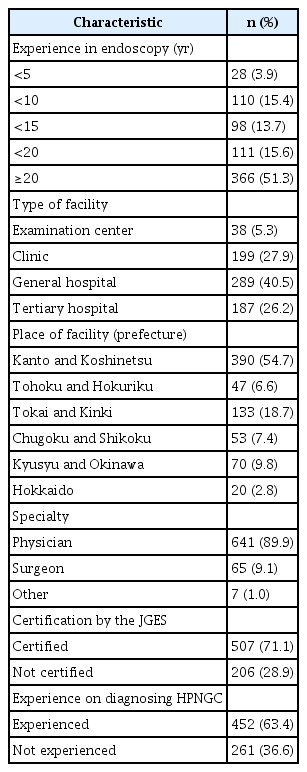
Valid responses were obtained from 713 endoscopists during the study period. The basic attributes of these endoscopists are listed in Table 2. Twenty-eight (3.9%) were trainees with less than five years of endoscopic experience, while 366 (51.3%) were skilled endoscopists with more than 20 years of experience. Most of the endoscopists worked in clinics, general hospitals, and tertiary hospitals, and only 38 (5.3%) worked in examination centers. Most of the endoscopists who participated in the study specialized in internal medicine (641, 89.9%). A total of 507 endoscopists (71.1%) were JGES-certified. Additionally, 261 (36.6%) endoscopists had never detected HPNGC. The response rates were compared to the JGES councilors and the population in each region to identify any regional differences in the number of valid responses, and no significant differences were found (Supplementary Fig. 1).
Differences in experience in diagnosing HPNGC based on the endoscopists’ background
We investigated whether there were differences in the experience in diagnosing HPNGC based on the endoscopists’ background. Interestingly, trainees with less than five years of endoscopic experience were more likely to detect HPNGC (Fig. 1A). There were no differences in the experience in diagnosing HPNGC based on the size and location of the institution (Fig. 1B, C). Being a physician or surgeon was not associated with experience in identifying HPNGC (Fig. 1D). In contrast, JGES-certified endoscopists had more experience in diagnosing HPNGC than nonspecialists (66.0% vs. 53.4%) (Fig. 1E). We then compared awareness, proactiveness, and interest scores with the endoscopists’ background. Endoscopists with fewer years of endoscopic experience had lower awareness scores than endoscopists with more years of experience (p<0.05) (Fig. 2A). Awareness and proactiveness scores were higher for endoscopists working in tertiary hospitals than for those working in clinics (p<0.05) (Fig. 2B). Differences in affiliation location did not affect any of the scores (Fig. 2C). Endoscopists in internal medicine had higher awareness scores than those in surgical medicine (p<0.05) (Fig. 2D). JGES-certified endoscopists had higher awareness and proactiveness scores than nonspecialists (p<0.001 and p=0.005, respectively) (Fig. 2E).
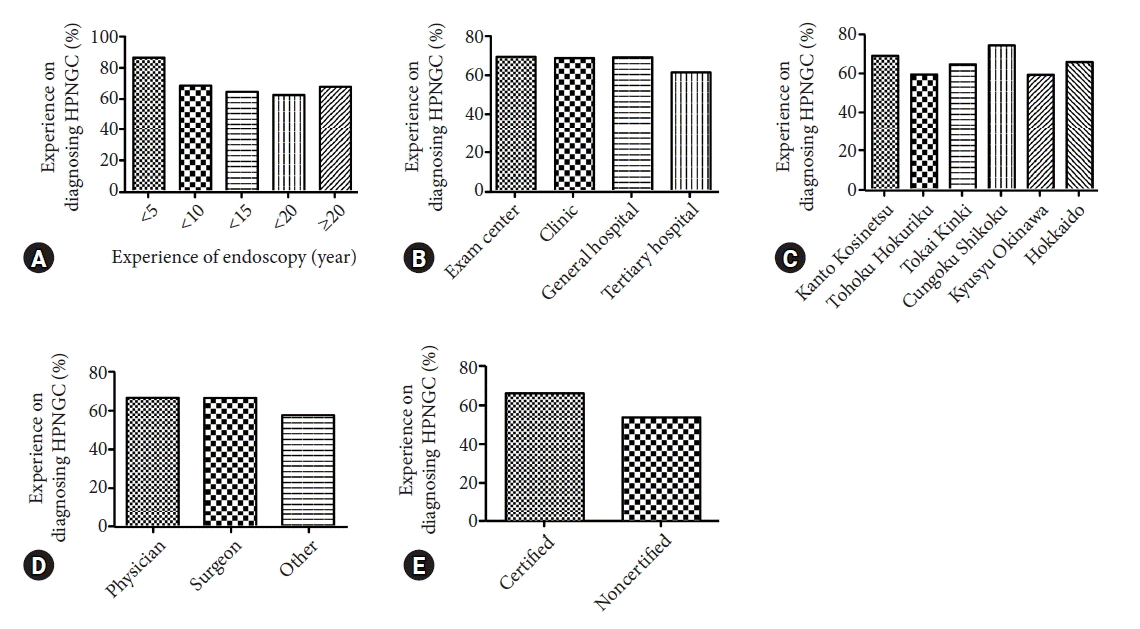
Relationship between the endoscopist background and the Helicobacter pylori-naïve gastric cancer (HPNGC) detection rate. (A) Relationship with experience in endoscopy (years). (B) Relationship with the size of the endoscopist's facility. (C) Relationship with the location (prefecture) of the facility where the endoscopist is affiliated. (D) Relationship with the endoscopist's area of expertise. (E) Relationship with whether the endoscopist obtained the Japan Gastroenterological Endoscopy Society certification as an endoscopist.
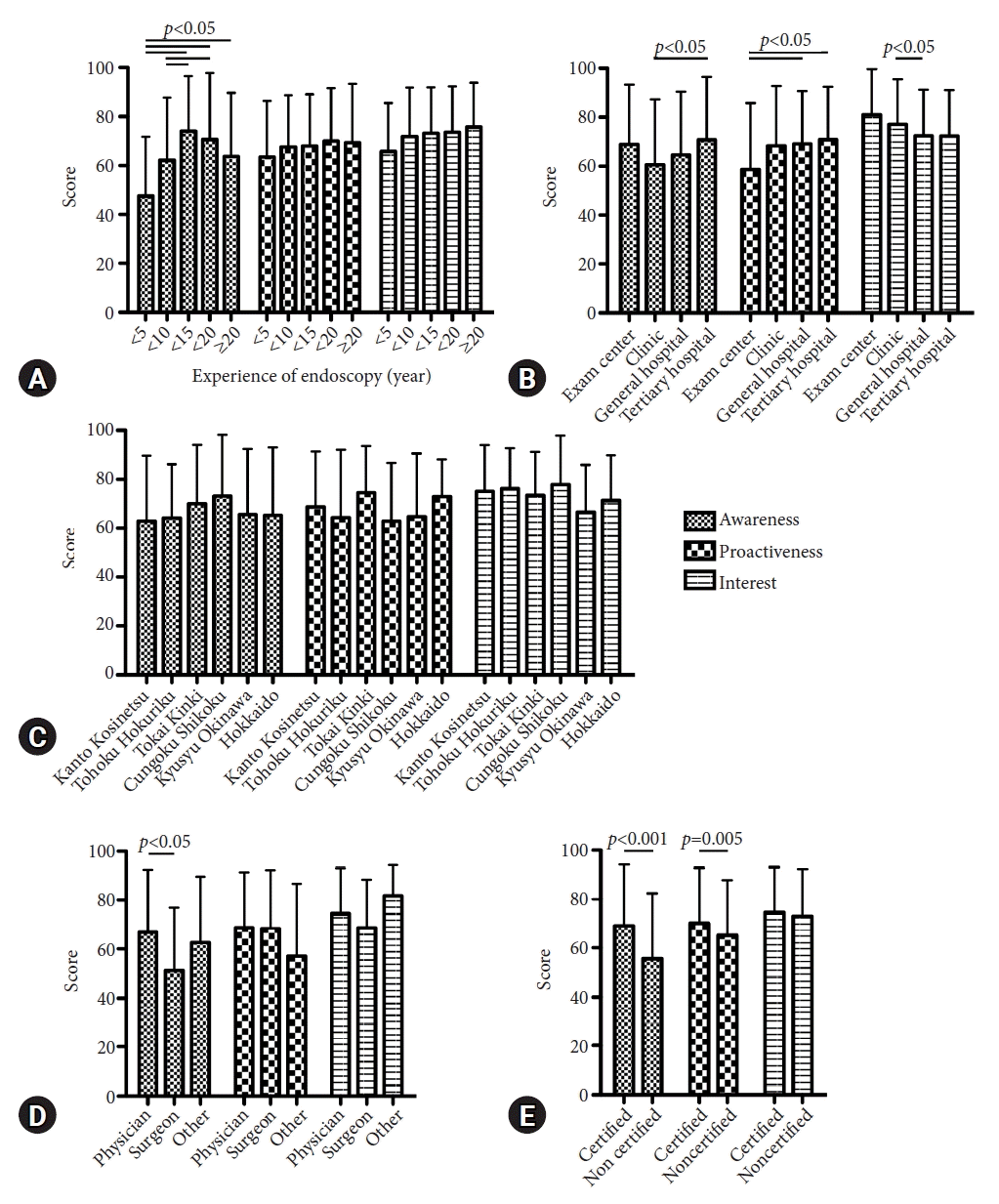
Association of endoscopist background with awareness, proactiveness, and interest scores. (A) Relationship with experience in endoscopy (years). (B) Relationship with the size of the endoscopist's facility. (C) Relationship with the location (prefecture) of the facility where the endoscopist is affiliated. (D) Relationship with the endoscopist’s area of expertise. (E) Relationship with whether the endoscopist obtained the Japan Gastroenterological Endoscopy Society certification.
Predictors of the detection of HPNGC
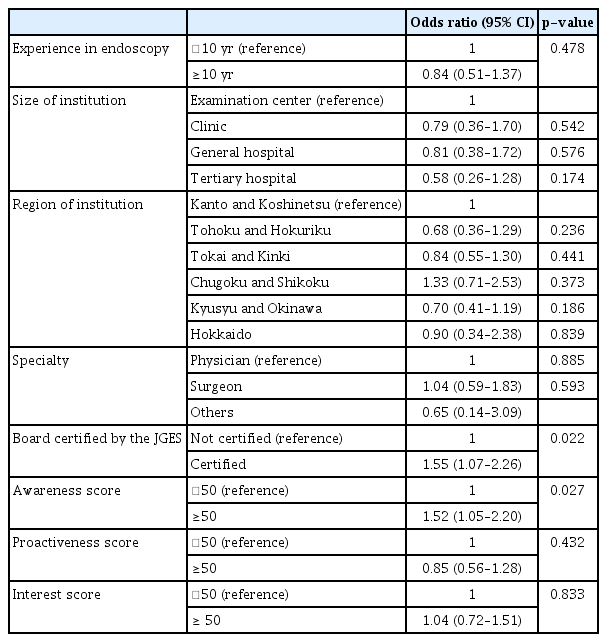
We constructed a predictive model for the experience in diagnosing HPNGC using a multiple regression model. Logistic regression analysis was performed to determine whether the backgrounds of all the endoscopists and awareness, proactiveness, and interest scores were explanatory variables and potential predictors of experience in diagnosing HPNGC. Consequently, being a JGES-certified endoscopist and having a high awareness score were found to be significant predictors of HPNGC detection (OR, 1.55; 95% CI, 1.07–2.26; p=0.022 and OR, 1.52; 95% CI, 1.05–2.20; p=0.027, respectively) (Table 3).
PCA for components of the awareness and interest score
Finally, we attempted to determine the more important factors among the components of the awareness and interest scores. The awareness score was subdivided into scores for GA-FG, raspberry-like tumors, and PSRCC, and added to the analysis. For the interest score, we added factors like attending scientific meetings and lectures hosted by academic societies or using web media such as article searches, social network services, e-mail newsletters, and blog articles. The background, awareness, proactiveness, and interest scores of all the endoscopists were represented two-dimensionally using PCA, and the weights of each component of the awareness and interest scores were visualized (Fig. 3F). Among the components of the awareness score, no specific trend was found for the scores related to GA-FG and raspberry-like tumors (Fig. 3A, B). In contrast, the score related to PSRCC was significantly higher than the scores related to the endoscopists’ interest scores (Fig. 3C). Among the components of the interest score, attending scientific meetings was more strongly associated with factors that had a positive impact on the endoscopists’ HPNGC detection rate (Fig. 3D, E).
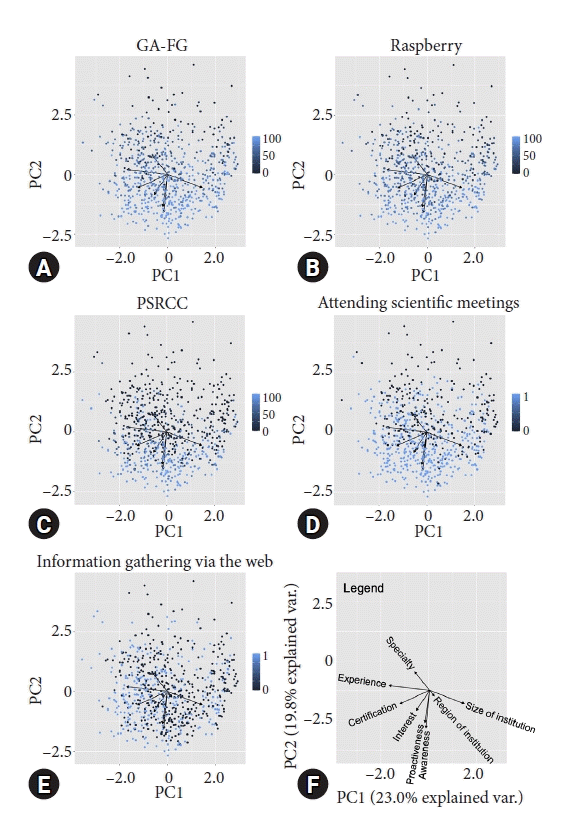
Principal component analyses (PCAs) of endoscopists’ backgrounds, and awareness, proactiveness and interest scores. Each dot indicates the position of the endoscopist on the PCA map. The lighter the blue color of the dots, the higher the score. (A) PCA map classified by scores related to gastric adenocarcinoma of the fundic-gland type (GA-FG) among the components of the awareness score. (B) PCA map classified by scores related to raspberry-like tumors among the components of the awareness score. (C) PCA map classified by scores related to pure signet ring cell carcinoma (PSRCC) among the components of the awareness score. (D) PCA map classified by scores related to attendance at scientific meetings among the components of the interest score. (E) PCA map classified by the scores related to web-based information collection among the components of the interest score. (F) PCA map legend. The size and direction of the black arrows indicate the contribution of each factor.
DISCUSSION
HPNGC is difficult to diagnose without a thorough understanding of its endoscopic characteristics. Since HPNGC is often missed during endoscopy screening, it is important to educate endoscopists about identifying HPNGC. However, no studies have investigated the relevance of educating endoscopists about HPNGC and whether it truly improves the identification rate of HPNGC. This study was the first to explore quality indicators using experience in diagnosing HPNGC as an outcome.
The results of this cross-sectional study with more than 700 endoscopists across Japan showed that JGES-certified specialists were more likely than nonspecialists to diagnose HPNGC. However, differences by region and size of the institution they worked in were not found to be directly related to HPNGC detection. Interestingly, the number of years of endoscopic experience was not directly associated with HPNGC detection. The results suggest the importance of obtaining certification from the JGES. Based on these results, we believe that the endoscopic specialist system operated by the JGES has universally ensured the quality of the endoscopists without creating regional disparities.
Certification by the JGES, awareness scores, and interest scores were all shown to be significant variables in our prediction model for experience in diagnosing HPNGC. However, the proactiveness score was not a significant variable. These results suggest that to improve the HPNGC detection rate, it is important to receive education especially from related societies and to become a JGES-certified endoscopist.
Furthermore, the PCA results indicate that it is important to improve our understanding of PSRCC among the HPNGCs. Surprisingly, PSRCC has low awareness and high interest despite being the classic HPNGC. As previously reported, PSRCC is the most frequently detected HPNGC, and it is important to educate the public about this common disease. It has also been suggested that scientific meetings and lectures play a significant role in gathering information about HPNGC. We believe that it is necessary to further educate endoscopists by providing accurate information about HPNGC, especially through related academic societies.
This study had several limitations. Firstly, the study may have selection bias. Most endoscopists in this study had more than 10 years of endoscopic experience and were JGES-certified, leading to a cohort with fewer less-experienced endoscopists. Secondly, this was a questionnaire-based cross-sectional study, which may have information bias, especially recall bias. Experience in diagnosing HPNGC, which was the primary outcome, was also based on the assumption of recall; therefore the results related to HPNGC detection may be overestimated. However, it is difficult to investigate the association between HPNGC awareness and interest scores and HPNGC detection in a prospective or retrospective observational study, and a cross-sectional questionnaire-based study design was employed.
In conclusion, candidates for quality indicators of HPNGC detection were found to be JGES certification, high awareness of HPNGC (especially PSRCC), and high interest in HPNGC. Additionally, participation in related conferences is an important tool for gathering information on HPNGC.
Supplementary Material
Supplementary Fig. 1. Councilor of Japan Society of Gastroenterological Endoscopy (JGES) and population distribution in Japan.
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.167.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: FI, TH, HU, YM; Data curation: FI, TH, HU YM; Formal analysis: FI, SS; Investigation: FI, SS; Methodology: FI, SS; Software: FI; Supervision: SS; Validation: TH, HU, YM; Visualization: FI, SS; Writing–original draft: FI; Writing–review & editing: all authors.