Necessity of pharyngeal anesthesia during transoral gastrointestinal endoscopy: a randomized clinical trial
Article information
Abstract
Background/Aims
The necessity for pharyngeal anesthesia during upper gastrointestinal endoscopy is controversial. This study aimed to compare the observation ability with and without pharyngeal anesthesia under midazolam sedation.
Methods
This prospective, single-blinded, randomized study included 500 patients who underwent transoral upper gastrointestinal endoscopy under intravenous midazolam sedation. Patients were randomly allocated to pharyngeal anesthesia: PA+ or PA– groups (250 patients/group). The endoscopists obtained 10 images of the oropharynx and hypopharynx. The primary outcome was the non-inferiority of the PA– group in terms of the pharyngeal observation success rate.
Results
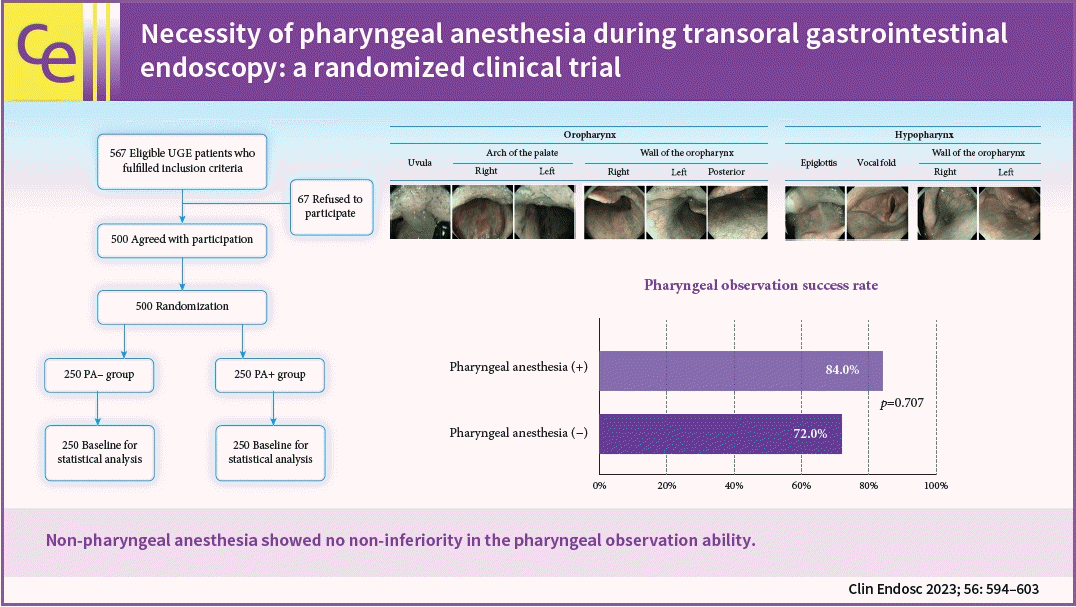
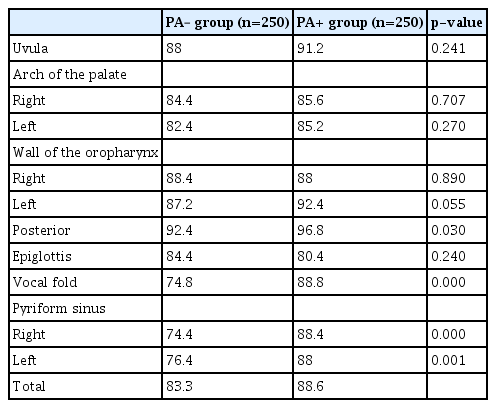
The pharyngeal observation success rates in the pharyngeal anesthesia with and without (PA+ and PA–) groups were 84.0% and 72.0%, respectively. The PA– group was inferior (p=0.707, non-inferiority) to the PA+ group in terms of observable parts (8.33 vs. 8.86, p=0.006), time (67.2 vs. 58.2 seconds, p=0.001), and pain (1.21±2.37 vs. 0.68±1.78, p=0.004, 0–10 point visual analog scale). Suitable quality images of the posterior wall of the oropharynx, vocal fold, and pyriform sinus were inferior in the PA– group. Subgroup analysis showed a higher sedation level (Ramsay score ≥5) with almost no differences in the pharyngeal observation success rate between the groups.
Conclusions
Non-pharyngeal anesthesia showed no non-inferiority in pharyngeal observation ability. Pharyngeal anesthesia may improve pharyngeal observation ability in the hypopharynx and reduce pain. However, deeper anesthesia may reduce this difference.
INTRODUCTION
Patients usually experience severe discomfort during transoral upper gastrointestinal endoscopy (UGE) because of the pain and strong gag reflex when the endoscope passes through the pharynx.1-3 Topical pharyngeal anesthesia, such as lidocaine (spray or viscous solution), reduces transoral UGE-induced pain and the gag reflex.4-7
According to a survey report, 0.0028% of UGE-related complications were due to pretreatment, whereas 0.00005% was fatal. The culprit drugs were sedative/analgesic-related and pharyngeal anesthesia-related in 46.5% and 8.3% of the cases, respectively.8 Thus, sedation/analgesic-related adverse events were more common than those related to pharyngeal anesthesia. However, sedation alleviates anxiety and discomfort in patients and improves their receptiveness and satisfaction with UGE.9 Although the frequency of side effects of lidocaine is lower, anaphylactoid reactions and lidocaine poisoning caused by increased blood concentration resulting in death have been reported.10 Therefore, the side effects of lidocaine are not negligible. Several studies on the use of pharyngeal anesthesia under sedation with propofol have reported that pharyngeal anesthesia does not reduce the amount of propofol, improve endoscopists’ satisfaction, or improve pharyngeal discomfort or patient satisfaction.11-14 However, at present, benzodiazepines are more commonly administered than propofol to obtain optimal sedation during UGE,9 as they do not require management by an anesthesiologist.
Therefore, the necessity of pharyngeal anesthesia in UGE under sedation with benzodiazepines is controversial. As reported previously, pharyngeal anesthesia before UGE improved endoscopy ease and patient tolerance.3 However, the number of cases was small in each study and the sedation method was not consistent. With advances in endoscopic instruments, narrow-band imaging has improved early superficial pharyngeal carcinoma detection and resulted in a better prognosis.15-17 Therefore, the importance of pharyngeal observation is increasing. However, endoscopic pharyngeal observation is difficult; careful observation of the pharynx while avoiding the gag reflex is essential to reduce the incidence of undetected cancers.
Experienced doctors can observe 86% to 90% of the sites without blind spots.18,19 However, there are no reports on whether pharyngeal anesthesia affects pharyngeal observation in patients sedated with benzodiazepines. Therefore, we conducted a prospective, single-blinded, randomized clinical trial to compare the groups undergoing transoral endoscopy with and without pharyngeal anesthesia under sedation with benzodiazepines. In addition to evaluating the degree of pain, we assessed pharyngeal observation quality between the two groups. This study aimed to prove the hypothesis that pharyngeal anesthesia is not required in patients sedated with benzodiazepines. If the group not receiving pharyngeal anesthesia was not inferior to the group receiving it, pharyngeal anesthesia was considered unnecessary. Therefore, a non-inferiority trial was deemed appropriate for testing this hypothesis. Randomized controlled trials evaluating patients’ pain and pharyngeal observation ability with pharyngeal anesthesia compared to those without pharyngeal anesthesia under sedation with benzodiazepines are required.
METHODS
Study design
This single-center, prospective, single-blinded, randomized study was conducted at Kanazawa University Hospital, Ishikawa, Japan. Patients were randomized into groups, with or without pharyngeal anesthesia, using computer-generated numerical codes by the information manager (simple randomization, allocation ratio 1:1).
After adopting the pharyngeal anesthesia protocols described below, the first image upon insertion of the endoscope was captured, followed by six and four endoscopic images of the oropharynx and hypopharynx, respectively. The last image was captured before insertion into the esophagus. Overall, 12 narrow-band imaging images were acquired for each patient. We also measured the time from the first image to insertion into the esophagus. Once it was impossible to obtain images owing to the continuous gag reflex, the endoscope was inserted into the esophagus. Hence, the first and last images were used only for time measurement.
The physicians were required to undergo more than half a year of pharyngeal observation training prior to participating in this study. Two endoscopists (TH and YA) assessed whether the 10 images were appropriate (Fig. 1). They were blinded to the allocation group and independent of the examining physicians. According to previous reports,18,19 the definition of an appropriate image requires the fulfillment of three criteria. First, an image was captured at an appropriate location. Second, we focused on the images. Third, the mucus was removed, and the pharyngeal mucosa color in the images was evaluated. Consensus was reached upon discussion among expert endoscopists in cases of disagreement.
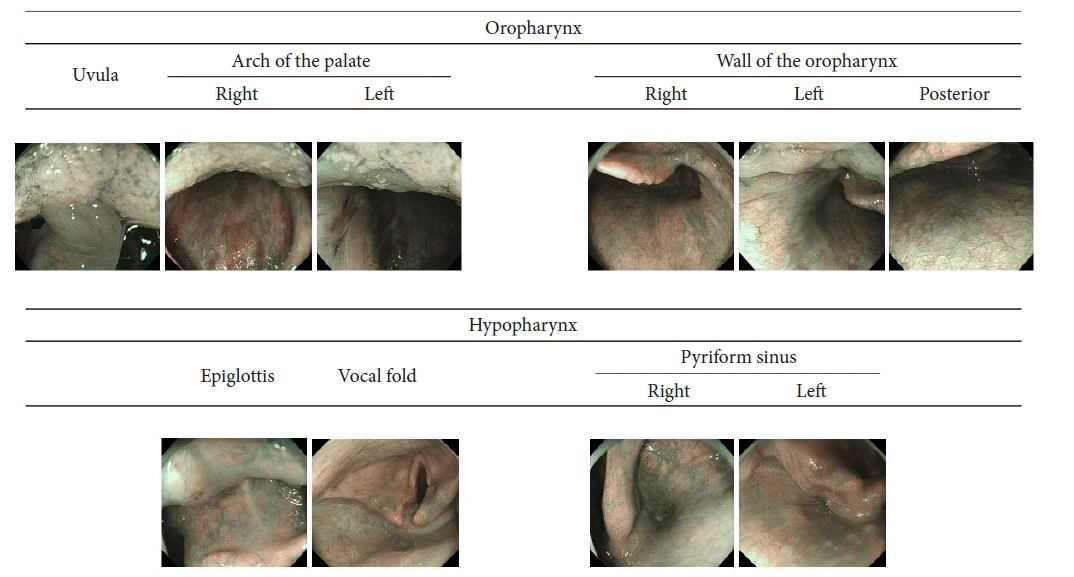
The 10 images of the oropharynx and hypopharynx. The definition of a high-quality image required three criteria: (1) the image was taken at the appropriate location; (2) the image was focused; and (3) mucus had been removed, and it was possible to evaluate the color of the pharyngeal mucosa.
The following data were recorded for each patient after endoscopy: age, sex, body mass index (BMI), performance status (PS) of the Eastern Cooperative Oncology Group, drinking and smoking habits, and the number of endoscopic experiences. Subjective pain symptoms were evaluated using an 11-point visual analog scale (VAS), with 10 points indicating the most severe pain and zero indicating no pain. Data were collected using a questionnaire after they were completely awake.
Patients
Patients aged ≥20 years who underwent transoral UGE under intravenous sedation with midazolam between July 2019 and July 2020 were asked to provide written informed consent before endoscopy. We excluded patients who refused to participate in the study, had previously undergone surgical or endoscopic mucosal resection for pharyngeal or laryngeal cancer because of pharynx or larynx removal, or had a severely damaged, difficult-to-evaluate pharynx. Other exclusion criteria were lesions in the pharynx requiring biopsy or magnified observation; emergency endoscopy for gastrointestinal bleeding or food impaction, lidocaine allergy, psychosis, or psychotic symptoms; and those deemed unfit to participate in the study by the investigator.
Anesthesia protocols
Patients who received pharyngeal anesthesia (PA+ group) were asked to swallow 40 mg (five puffs) of lidocaine (Xylocaine Pump Spray 8%; AstraZeneca), whereas patients who did not receive pharyngeal anesthesia (PA– group) were not asked to swallow anything. The examining physicians were blinded to the randomization. To maintain a single-blind format, they entered the room only after the administration of premedication, including pharyngeal anesthesia.
Endoscopic examinations
All endoscopic procedures were performed using a magnifying endoscope (GIF-H290Z; Olympus Medical Systems) equipped with a hood attachment (MAJ-1990; Olympus Medical Systems). The video endoscopy system used in this study comprised a video processor (EVIS LUCERA ELITE CV-290; Olympus Medical Systems) and light source (EVIS LUCERA ELITE CLV-290SL; Olympus Medical Systems). Pharyngeal anesthesia was administered as described in “Anesthesia protocols.”
The procedures were conducted under sedation with midazolam (midazolam injection; Sandoz). It was administered at 0.05 mg/kg or with reference to the information regarding previous benzodiazepine consumption if it existed. This amount was determined by the examining physicians who were blinded to the allocation results. The pharynx was assessed at the beginning of each examination and standard endoscopy was performed after pharyngeal examination. In cases where pharyngeal lesions were detected, they were evaluated by magnification after pharyngeal examination because it is necessary to measure the pharyngeal observation time. The number of gag reflex events was counted during pharyngeal observation by the examining physicians and promptly recorded after endoscopy.
Outcome measures
The main outcome was the difference in pharyngeal observation success rate between the PA– and PA+ groups. We defined successful pharyngeal observation as being able to take appropriate images in eight or more of the 10 sites within 120 seconds since experienced endoscopists observed the pharynx; an average of 9.018 and 8.619 might be observed out of 10 sites. In a previous study, when still images and videos were compared in cases with ≥8 imaging sites, almost all test sites could be confirmed in the video. However, with ≤7 imaging sites, significantly lower sites could be examined.20 Moreover, the assessments of the two evaluator endoscopists were significantly different. Therefore, pharyngeal observation could not be considered successful.
Hence, the quality of pharyngeal observations may improve when the observation time is longer. However, a longer time results in a significant delay in examination, which is unacceptable in routine screening. There is no absolute standard for pharyngeal observation time; therefore, we postulated that 15 UGEs would be performed in 3 hours (12 minutes/UGE), considering a reported average time for pharyngeal observation of 69 seconds.18 If the examination time/patient was extended by 51 seconds, only 14 UGEs/hour would be possible, which is not acceptable. Hence, a successful pharyngeal observation can be completed within 69+51=120 seconds.
The secondary outcomes were as follows: (1) a difference in the number of observable pharynx sites; (2) a difference in endoscopy-associated pain; (3) a difference in the pharyngeal observation time; (4) subgroup analysis of the level of sedation by Ramsay score; (5) adverse effects of lidocaine, including decreased SpO2 (<90% or decrease of more than 4% for <94%), decreased blood pressure (systolic blood pressure <90 mmHg), and bradycardia (<60 bpm or decrease of more than 10%)21; and (6) the percentage of suitable quality images obtained at the 10 prescribed points. The Ramsay score is a numeric score from 1 to 6 based on the responsiveness of the patient (1: Patient is anxious and agitated or restless, or both. 2: The patient is cooperative, oriented, and tranquil. 3: The patient responds to commands only. 4: The patient exhibits a brisk response to a light glabellar tap or loud auditory stimulus. 5: The patient exhibits a sluggish response to a light glabellar tap or loud auditory stimulus. 6: Patient exhibits no response.).22
Statistical analysis
The non-inferiority of the PA– group to the PA+ group was examined. From a reanalysis of our previous study,19 an 81.8% success rate was confirmed when pharyngeal observation success was defined as above. In this study, we referred to the US Food and Drug Administration standard23; therefore, the non-inferiority threshold [Δ] was set to 10%. It was estimated that at least 468 cases were required to detect statistically significant differences, with a type I error rate of 0.025 (one-sided test) and statistical power of 80%. Considering that that the dropout rate was estimated 5% to 10% by consulting a statistician, we determined that it was necessary to include a total of 500 cases.
Continuous variables are expressed as mean (standard deviation), and comparisons between groups were performed using Student t-test or the Mann-Whitney U test (not approximately normally distributed). Categorical variables are expressed as percentages, and comparisons between groups were performed using Fisher’s exact test. Statistical significance was set at p<0.05. Only researchers collected and aggregated the data. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc.) and Stata ver. 17 (StataCorp.).
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki, the Clinical Trials Act, ethical guidelines for medical and health research involving human subjects, and all other applicable laws and guidelines in Japan. The study protocol was approved by the Institutional Review Board at Kanazawa University (approval number: CRB4180005) on April 25, 2019. This study was registered at the Japan Registry of Clinical Trials (jRCTs041190031).
RESULTS
Figure 2 shows a flow chart of patient selection and allocation. A total of 500 patients were enrolled and randomly allocated to the PA– and PA+ groups; 250 in each group. The patient demographics and clinical characteristics are shown in Figure 3. The number of patients in both groups was similar. No statistically significant differences were observed in age, sex, height, weight, BMI, PS, drinking and smoking habits, number of endoscopic procedures, and the Ramsay score at sedation.

Patients demographics and clinical characteristics and subgroup analysis of patients’ characteristics. CI, confidence interval.
The agreement rate between the two endoscopists was 97.2% and the kappa statistic was 0.916.
The pharyngeal observation success rate in the PA– group was 72.0% (95% confidence interval [CI], 66.4%–77.6%), whereas that in the PA+ group was 84.0% (95% CI, 79.5%–88.5%). The difference was –19.2% to –4.8% (p=0.707, non-inferiority), and the non-inferiority of the PA group was not demonstrated (Table 1). In the PA– group, one oropharyngeal cancer, and one hypopharyngeal cancer were detected. Table 2 shows the differences in situations during endoscopy. The PA– group was inferior to the PA+ group in the number of observable parts (0–10) (8.33±2.32vs. 8.86±1.94, p=0.006), pharyngeal observation time (67.2±33.7 vs. 58.2±27.1 seconds, p=0.001), pain evaluated by VAS (1.21±2.37 vs. 0.68±1.78, p=0.004), and in the number of gag reflexes (3.83±3.15 vs. 2.11±2.49, p<0.001). There were no significant differences in adverse events, decrease in SpO2 (12.0% vs. 10.0%, p=0.475), decrease in blood pressure (1.2% vs. 0.4%, p=0.623), or bradycardia (0.4% vs. 1.2%, p=0.623) between the two groups.

Non-inferiority test of the difference of pharyngeal observation success rate (based on a risk α of 0.025 [one-sided test], a statistical power of 80%, and a non-inferiority threshold [Δ] of 0.1)
Table 3 shows the percentages of suitable quality images obtained at the 10 prescribed points. The percentage of suitable quality images at the posterior wall of the oropharynx (92.4% vs. 96.8%, p=0.030), vocal fold (74.8% vs. 88.8%, p<0.001), and right (74.4% vs. 88.4%, p<0.001) and left pyriform sinuses (76.4% vs. 88.0%, p=0.001) were significantly lower in the PA– than in the PA+ group. No significant differences were observed at any of the other parameters.
Subgroup analysis of the lower level of sedation (Ramsay score <5 in 217 patients) revealed that the pharyngeal observation success rate (61.6% vs. 81.9%, p=0.001), number of pharyngeal observation sites (7.88±2.55 vs. 8.89±1.89, p=0.001), and number of gag reflexes (3.97±3.07 vs. 2.70±2.73, p=0.001) were significantly lower in the PA– than in the PA+ group. However, non-significant differences were found in pain evaluated by VAS (1.85±2.91 vs. 1.27±2.42, p=0.113) and pharyngeal observation time (67.0±32.9 vs. 59.8±31.9 seconds, p=0.104) (Table 1). The subgroup analysis of the higher level of sedation (Ramsay score ≥5 in 283 cases) revealed that pain evaluated by VAS (0.70±1.66 vs. 0.25±0.77, p=0.005), pharyngeal observation time (67.3±34.4 vs. 56.7±23.1 seconds, p=0.003), and the number of gag reflexes (3.70±3.22 vs. 1.68±2.20, p<0.001) were significantly lower in the PA– than in the PA+ group. However, a non-significant difference between the two groups was found in the pharyngeal observation success rate (80.4% vs. 85.5%, p=0.255) and the number of pharyngeal observation sites (8.70±2.05 vs. 8.83±1.97, p=0.562) (Table 1). Figure 3 shows the subgroup analysis of patients’ characteristics, such as sex, age (over or under 70), BMI (over or under 25), alcohol intake (over or under one time/week), smoking status, PS (0 or >1), antihypertensive or psychotropic drugs use, Ramsay score (over or under 5), and the number of examinations (over or under 5). In comparing the BMI and Ramsay scores, the p values were <0.05.
DISCUSSION
To the best of our knowledge, this is the first study to evaluate pharyngeal observation ability in patients undergoing transoral UGE under sedation with or without administration of topical pharyngeal anesthesia. Our study revealed that non-inferiority of non-pharyngeal anesthesia was not demonstrated in terms of pharyngeal observation success rate, and the percentage of suitable quality images at the posterior wall of the oropharynx, vocal fold, and pyriform sinus was significantly inferior. However, in the subgroup analysis of higher levels of sedation, there were almost no differences in the pharyngeal observation success rate between patients with and without pharyngeal anesthesia.
However, in the ad hoc analysis, the PA+ group had a significantly higher pharyngeal observation success rate than the PA– group (p=0.001). Naturally, it is not a preset test; however, the PA+ group may have better pharyngeal observation ability than the PA– group for patients who were administered intravenous anesthesia with midazolam.
The PA+ group was significantly superior to the PA– group in terms of the number of observable pharyngeal parts (0.53 parts), observation time (9.0 seconds), pain measured using VAS (0.53 points), and the number of gag reflexes (1.72 times) owing to the advantages of pharyngeal anesthesia using lidocaine. These differences were presumably small absolute numerical values between the groups. However, these are clinically important facts for endoscopists, who focus on reducing patients’ pain as much as possible. Pharyngeal anesthesia may be tolerated by weighing these differences against side effects.
In the subgroup analysis of each pharyngeal site, the PA– group was statistically inferior to the PA+ group in terms of observation of the posterior wall of the oropharynx, epiglottis, vocal fold, and right and left pyriform sinuses. This difference can be attributed to multiple comparisons. It is suspected that the effect of anesthesia on the hypopharynx may be stronger in the PA+ group; however, more cases need to be evaluated to prove the theory. Further studies on the relationship between the extent of hypopharyngeal anesthesia and pharyngeal observation ability are desirable because the pyriform sinus is the predilection site for pharyngeal cancer.
In the subgroup analysis for each variable, obese patients (BMI ≥25 kg/m2) and patients with low sedation levels (Ramsay score <5) may have had low pharyngeal observation success rates in the PA group. To clarify these differences, it is necessary to include a larger number of cases.
Detailed subgroup analysis of each sedation level revealed that the pharyngeal observation success rate and the number of observable sites were low in the PA group at low sedation levels (Ramsay score <5). However, these differences were not observed at high sedation levels (Ramsay score ≥5). Based on our findings, it is reasonable to presume that deep sedation reduces the disadvantage of not using pharyngeal anesthesia. This would be beneficial for patients who cannot use pharyngeal anesthesia because of allergies. Although the possibility of chance cannot be ruled out by multiple analyses, as deep sedation with propofol has shown that pharyngeal anesthesia is unnecessary, the deeper the sedation level, the lesser the pharyngeal anesthesia that might be required. No data have clarified this hypothesis. Further studies based on this hypothesis are required.
In the deep sedation group, pharyngeal anesthesia may further reduce pain and pharyngeal observation time. Although difficult to explain theoretically, this may be because pharyngeal anesthesia reduces pain and the difficulty in observation. Alternatively, considering that there is a similar tendency without significant difference in the low sedation group, it may not be relevant to sedation. Large-scale studies are necessary to further investigate this.
Diagnostic transnasal endoscopy has been proven to be more tolerable than the transoral approach.24,25 Furthermore, during the coronavirus disease 2019 pandemic, transnasal endoscopy has proven to be beneficial in terms of less aerosol spreading.26 However, transoral endoscopy models have magnification and better image quality. Therefore, endoscopists prefer this approach.
This study had some limitations. First, it was conducted at a single institution. Second, it was single-blind because the patients knew whether pharyngeal anesthesia was administered. The PA group did not receive a placebo intervention because of the difficulty of creating a placebo with a flavor similar to that of lidocaine. Thus, the participants’ awareness of whether pharyngeal anesthesia was administered might have altered their mental state and affected the outcome. Third, our study did not evaluate the postcricoid area. The nasal Valsalva or sniffing position has been reported to improve examination in transoral endoscopy27,28; however, we did not adopt such methods. However, further studies are required to explore these methods. Fourth, no synthetic opioid analgesics such as pethidine were used as premedication. However, our findings are important for facilities that are unable to use them. Fifth, high-risk patients with pharyngeal cancer often have a history of alcohol consumption and may tolerate sedation. Such patients are considered more suitable for participation. Sixth, the definition of successful pharyngeal observation formulated by us may seem controversial among experts. The results of this study were based on two expert endoscopists’ opinions of endoscopic images, and the outcomes were based on subjective evidence without validation. To date, there has been no consensus among experts in this regard. Therefore, it is desirable to establish clear criteria for future clinical studies.
In conclusion, we found that not administering pharyngeal anesthesia did not demonstrate non-inferiority of pharyngeal observation ability. Pharyngeal anesthesia may improve pharyngeal observation ability, especially in the hypopharynx, and reduce patients' pain. However, this difference may be reduced by deeper anesthesia.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
The authors wish to thank Kazuki Nagai, Akihiro Seki, Hidetoshi Nakagawa, Takashi Nakashima, Hiroki Matsukawa, Shinya Yamada, Keita Kita, Koki Yamagata, Emi Aburao, Kiichiro Kaji, Gen Sugiyama, Kouki Nio, Hiroki Nomura, Masaemon Momonoi, Kosuke Satomura, Yuta Tamaru, Seiya Kamata, Kota Kitagawa, Toshikatsu Tamai, and all endoscopy medical staff at the Kanazawa University Hospital.
Author Contributions
Conceptualization: TH; Data curation: TH; Formal analysis: TH, SM, TTe, TTo; Investigation: YA, YT, MM, HT, HK, JS, NI, KK; Methodology: TH, TTe; Project administration: TH, TTe, EM; Supervision: SM, TTo, TY; Writing–original draft: TH; Writing–review & editing: all authors.