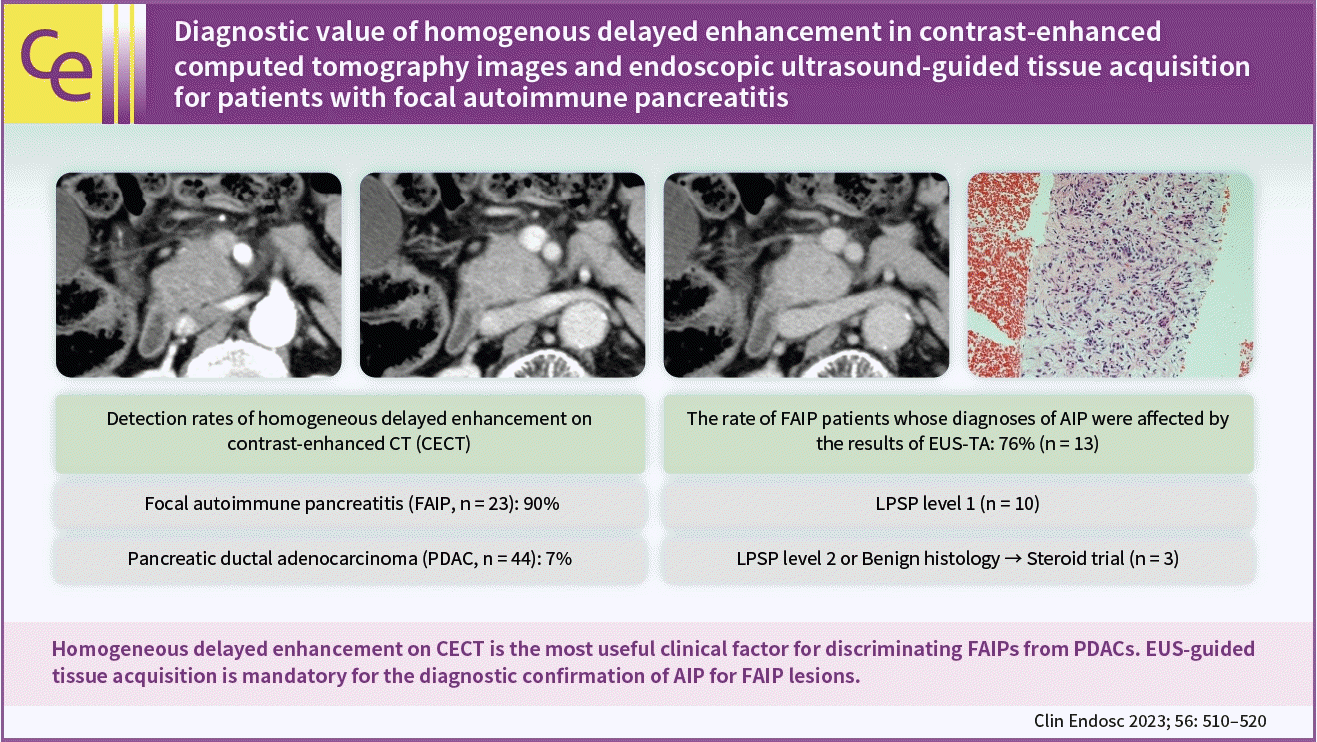
Diagnostic value of homogenous delayed enhancement in contrast-enhanced computed tomography images and endoscopic ultrasound-guided tissue acquisition for patients with focal autoimmune pancreatitis
Article information
Abstract
Background/Aims
We aimed to investigate (1) promising clinical findings for the recognition of focal type autoimmune pancreatitis (FAIP) and (2) the impact of endoscopic ultrasound (EUS)-guided tissue acquisition (EUS-TA) on the diagnosis of FAIP.
Methods
Twenty-three patients with FAIP were involved in this study, and 44 patients with resected pancreatic ductal adenocarcinoma (PDAC) were included in the control group.
Results
(1) Multivariate analysis revealed that homogeneous delayed enhancement on contrast-enhanced computed tomography was a significant factor indicative of FAIP compared to PDAC (90% vs. 7%, p=0.015). (2) For 13 of 17 FAIP patients (76.5%) who underwent EUS-TA, EUS-TA aided the diagnostic confirmation of AIPs, and only one patient (5.9%) was found to have AIP after surgery. On the other hand, of the six patients who did not undergo EUS-TA, three (50.0%) underwent surgery for pancreatic lesions.
Conclusions
Homogeneous delayed enhancement on contrast-enhanced computed tomography was the most useful clinical factor for discriminating FAIPs from PDACs. EUS-TA is mandatory for diagnostic confirmation of FAIP lesions and can contribute to a reduction in the rate of unnecessary surgery for patients with FAIP.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a unique form of chronic pancreatitis with infiltration of abundant lymphocytes. It is histologically classified into type 1 and 2.1,2 Type 1 AIPs are clinically known as a part of IgG4-related diseases3 and histologically shown to be lymphocytoplasmic sclerosing pancreatitis (LPSP), which was first reported by Kawaguchi et al.4 Type 1 AIPs account for the majority of AIPs in East Asia, including Japan, whereas type 2 AIPs, histologically involving granulocyte epithelial lesions, are common in Western countries.5,6 Although diffuse pancreatic enlargement (diffuse type) is a typical imaging feature of AIPs, some AIPs cause focal pancreatic enlargements or pancreatic mass lesions (focal AIP, FAIP), in which cases involving focal pancreatic lesions AIPs must be differentiated from pancreatic neoplasms, including pancreatic ductal adenocarcinoma (PDAC).
For differentiating AIPs from PDACs, several clinical studies have shown the usefulness of some serological markers and imaging findings.7-14 However, the accuracy of those findings to determine AIPs for patients with pancreatic focal lesions was unsatisfactory.7,9,10 Recently, endoscopic ultrasound-guided tissue acquisition (EUS-TA) has become important for the diagnosis of AIPs, especially due to the improvements in the method of processing specimens obtained from EUS-guided fine needle aspiration (EUS-FNA)15-17 and the advances in the EUS-FNA needles, namely, the development needles for EUS-guided fine needle biopsy (EUS-FNB).18,19 In addition, type 1 AIPs can be determined as being “definitive” by using only the histological findings of level 1 LPSPs obtained from EUS-TA based on the Internal Consensus Diagnostic Criteria (ICDC).5 Moreover, a diagnostic steroid trial can be used as an option for patients for whom EUS-TA provides no histological findings of malignancy despite lacking the histological diagnosis of level 1 LPSP.
Because some FAIPs are resected due to the difficulty in discriminating them from PDACs even now,20,21 the impact of EUS-TA on the diagnosis of FAIPs appears to be very large. However, the clinical implications of EUS-TA for FAIPs have not yet been fully studied. In addition, larger amounts of pancreatic tissue are required for diagnosing FAIPs than for PDACs when EUS-TA is used for histopathological diagnosis of pancreatic diseases. Therefore, it is mandatory to recognize pancreatic mass lesions as possible FAIPs using laboratory data and/or imaging findings obtained before performing EUS-TA. Thus, we conducted a retrospective study related to the diagnosis of FAIPs to clarify the following: (1) useful laboratory data or imaging findings for the recognition of possible FAIPs, and (2) the impact of EUS-TA on the diagnosis of FAIPs.
METHODS
Study population and data collection
Of the 80 patients retrospectively diagnosed with AIPs based on the ICDC by using the medical records of our medical center between January 2002 and December 2020, 23 patients with FAIPs were included in this study. FAIP was defined as a pancreatic lesion of an AIP located within one of the three pancreatic sites (pancreatic head, body, and tail) using contrast-enhanced computed tomography (CECT).
Laboratory data, imaging findings, and endoscopic findings were retrospectively collected and analyzed by two gastroenterologists (KY and SK). On the other hand, all histocytological findings in this study were prospectively determined and retrospectively analyzed.
Outcome measurements
We retrospectively evaluated the following: (1) the differences in the demographic, serological, and imaging findings between FAIP and PDAC, (2) the ability of EUS-TA to detect the histology of FAIP, and (3) the effects of EUS-TA on the diagnosis of FAIP using the ICDC. To evaluate (1), of the 125 patients who underwent surgery for their PDACs between January 2007 and July 2017 in our hospital, 44 with mass-forming PDAC localized in the pancreas for whom serum IgG4 and CA19-9 levels were preoperatively measured and who preoperatively underwent endoscopic retrograde pancreatography (ERP) were used as a control group for comparison.
Imaging studies
CECT was performed using a 64-detector row scanner (Aquillion; Toshiba). Dynamic CT was performed on all patients. The CT protocols for suspected AIPs in our hospital were composed of pre-contrast, arterial, portal, and delayed phases, and the scan delays from the injection of contrast medium to the start of the arterial, portal, and delayed phase imaging were determined to be 40, 60, and 120 seconds, respectively. The enhancement patterns on CECT were retrospectively detected with bare eyes by two gastroenterologists (KY and SK), and the detection of these findings was determined based on the consensus of the two gastroenterologists as mentioned above. When it was difficult to determine the enhanced pattern on CECT with bare eyes, we referred to the differences in CT values of the pancreatic lesion in each contrast-enhanced phase.
ERP procedures
All ERP procedures were performed following the Declaration of Helsinki. A TJF260V (Olympus Co.) was used as a backward oblique viewing endoscope to perform the ERP. A standard contrast injection technique was initially used with a 4-Fr cannula (PR-104Q-1 or PR-110Q-1; Olympus Co.). To obtain adequate images from pancreatography, contrast injection through the cannula was performed under fluoroscopic control with sufficient injection pressure. When a tapered narrowing of the main pancreatic duct (MPD) was visualized during ERP, MPD stenosis was defined as the MPD finding with a visualization of an upstream MPD; in contrast, MPD obstruction was defined as the finding without visualizing an upstream MPD despite an adequate pressure of contrast injection.
Procedures of EUS and EUS-TA for FAIPs
All EUS and EUS-TA procedures for FAIPs were performed in accordance with the Declaration of Helsinki. A GF-UCT260 (Olympus Co.) was used as an echoendoscope for EUS and EUS-TA, and EU-ME1 and EU-ME2 (Olympus Co.) ultrasonographic systems were used to process EUS images.
In our hospital, a 19-, 20-, 22-, or 25-G needle has been used to perform EUS-TA for suspected AIP lesions at the physician’s discretion, and EUS-FNB needles have been mainly used for the histological diagnosis of AIPs since 2017. For each needle pass, 10 to 20 strokes with negative pressure applied using a 20-mL syringe were made. Although at least three needle passes were usually performed during EUS-TA for possible FAIPs, additional passes were performed when the volumes of the specimens obtained via three needle passes were macroscopically inadequate.
All specimens obtained via EUS-TA for FAIPs were pushed out from the inside of the needles into a centrifuge tube containing 5 mL of 10% formalin solution using a small volume of saline or a needle stylet. These specimens were mainly processed using a cell-block method,22 and rapid onsite cytological evaluation was not performed for this study population.
Histocytological evaluations
For this study population, all histocytological diagnoses of specimens obtained through EUS-TA and surgically resected specimens were prospectively made via the consensus of two or more pathologists with specific expertise in gastrointestinal pathology (YN, TS, MU, and FF).
To assess the histology of the EUS-TA specimens, cell-block sections were prepared using a sodium alginate method and subjected to hematoxylin and eosin (H&E) and periodic acid-Schiff and Alcian blue stains as basic histological stains. When AIP was suspected based on clinical and histological findings obtained by H&E staining of EUS-TA specimens, immunostaining was performed using antibodies against IgG4 (HP6023; Millipore) and leukocyte common antigen (2B11+PD7/26; Nichirei). Elastica-Masson staining was performed to evaluate fibrosis and obliterative phlebitis. When there was a possibility of pancreatic malignancies, including PDACs, based on the histological findings obtained via H&E staining of the EUS-TA specimens, antibodies against Ki67 (MIB-1; Immunotech), P53 (DO-7; DAKO), and MUC1 (Ma695; Novocastra) were used for further evaluation of malignancy.
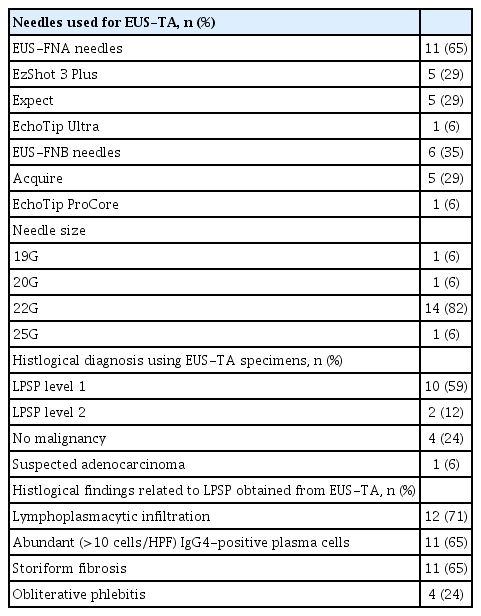
For EUS-TA and resected specimens, a diagnosis of LPSP was made based on the ICDC. Level 1 LPSPs were determined when three or more of the following four histological findings were met: (1) lymphoplasmacytic infiltration, (2) IgG4-positive plasma cell infiltration >10 counts/high-power field, (3) presence of storiform fibrosis, and (4) obliterative phlebitis.3 Level 2 LPSPs were determined when two of the four histological findings mentioned above were met.
Steroid trial for FAIPs
When a diagnosis of definitive AIP for patients with possible FAIPs cannot be made from several clinical and pathological examinations, including EUS-TA findings, despite no evidence of malignancy obtained through EUS-TA, a diagnostic steroid trial can be used as an option for the confirmation of definitive FAIPs based on the ICDC. In this study, this optional trial was conducted by orally administering 0.6 mg/kg/day of prednisolone for two weeks, followed by an evaluation of the morphological changes in the pancreatic focal lesion using CECT. When a focal pancreatic lesion was shown to decrease in size adequately or become obscure on re-examination with CECT after a steroid trial, a response to the steroid trial was confirmed.
Statistical analyses
Comparisons among the groups were performed using Student t-test or the Mann-Whitney U-test for continuous variables and the χ2-test or Fisher exact test for categorical variables. Multivariate analysis using multiple logistic regression was performed for the factors that showed a p-value of <0.05 by univariate analysis. Statistical significance was set at p<0.05. All statistical analyses were performed using IBM SPSS Statistics software program ver. 24.0 (IBM Corp.).
Ethical statements
This study was approved by the Sendai City Medical Center Institutional Review Board (registration number: 2020-0010).
RESULTS
Patient characteristics
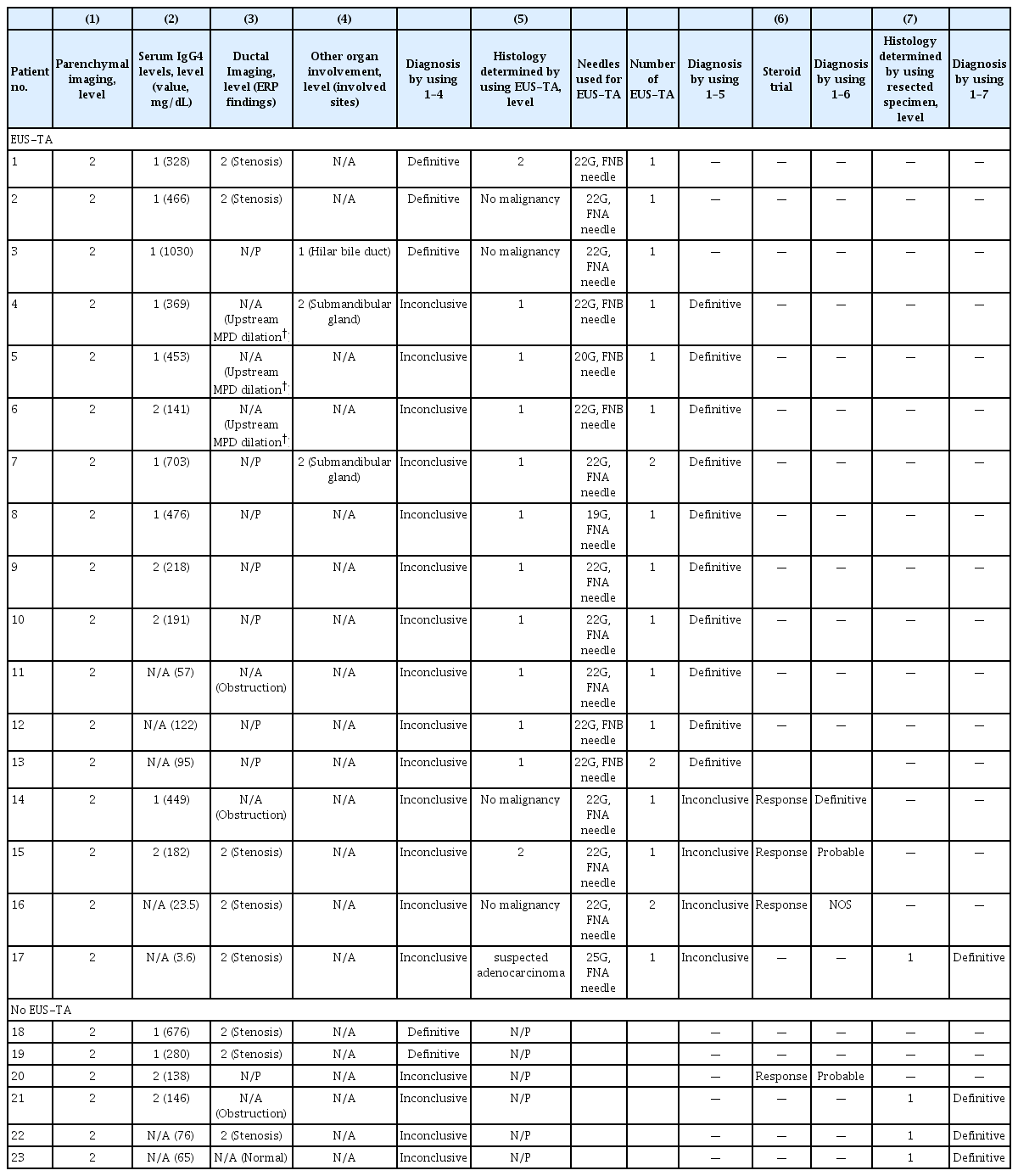
Table 1 shows the baseline characteristics of the patients with FAIP. The 23 patients with FAIPs in this study consisted of 16 men and seven women, with a mean age of 70.0±9.8 years. Serum IgG4 levels >135 mg/dL were detected in 16 patients (69.6%), and only three patients (13.0%) had other organ involvement (OOI). ERP was performed in 15 patients (65.2%), and EUS-TA was performed in 17 patients (73.9%). Based on the ICDC, 22 patients (95.7%) were diagnosed with type 1 AIPs (definitive type 1 AIP, 20; probable type 1 AIP, 2), the remaining patient was diagnosed with an AIP not otherwise specified (AIP-NOS), and no patients were found with type 2 AIPs in this study. With regard to the treatment of AIP lesions, 14 patients (60.9%) received steroid therapy, four patients underwent surgery, and the remaining five patients underwent surveillance without any treatment for their AIP lesions.
Differences in the demographic, laboratory, and imaging factors between FAIPs and PDACs
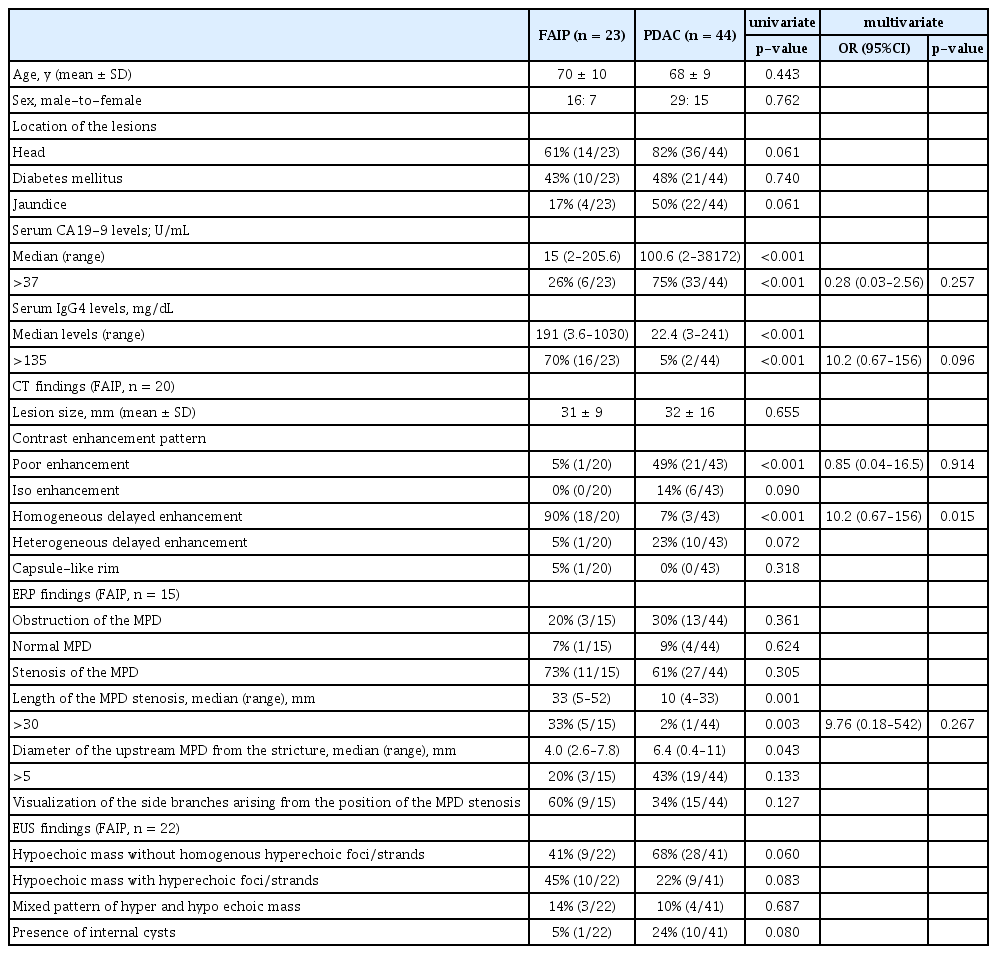
The demographic, laboratory and imaging factors for patients with FAIP and PDAC are compared in Table 2. For laboratory data, the rates of serum CA19-9 levels (>37 U/mL) and serum IgG4 levels (>135 mg/dL) were significantly different between the two groups (26% vs. 75%, p<0.001; 70% vs. 5%; p<0.001, respectively). Contrast enhancement patterns using a CT scan differed between the two groups, and homogeneous delayed enhancement (Fig. 1) was detected more frequently in the FAIP group (90% vs. 7%, p<0.001). On the other hand, a poor enhancement pattern was detected more frequently in the PDAC group (5% vs. 49%, p<0.001). In addition, MPD stenoses of >30 mm using ERP imaging were significantly more frequent in patients with FAIP (33% vs. 2%, p=0.003). There were no significant differences in EUS findings between the two groups.
Comparison of demographic, laboratory, and imaging factors for FAIP and PDAC patients at the initial visit
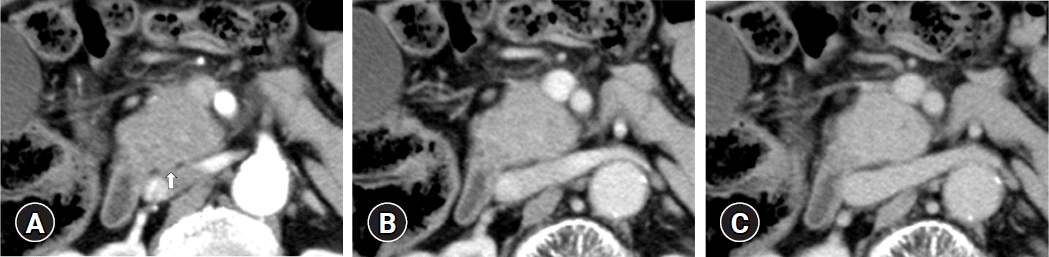
This patient (patient no. 1 in Table 4) was referred to our hospital due to a mass lesion in the pancreatic head. Using contrast-enhanced computed tomography, a focal mass lesion in the pancreatic head (arrow) which showed hypovascularity during the arterial phase (A) and homogeneously delayed enhancement during the portal (B) and delayed phases (C) was detected.
In addition, to narrow down the valuable factors to discriminate FAIP from PDAC, we performed a multivariate analysis using the above-mentioned five factors with a p-value <0.05 using univariate analysis. From the multivariate analysis results, only homogeneous delayed enhancement on CECT was shown to be a significant factor indicative of FAIP compared to PDAC (p=0.015, Table 2).
Results from EUS-TA for patients with FAIPs
The EUS-TA results in patients with FAIP are listed in Table 3. As mentioned above, 17 of the 23 patients with FAIP (73.9%) underwent EUS-TA for FAIP lesions owing to the confirmation of AIP. Conventional EUS-FNA needles were used in 11 patients (64.7%), and EUS-FNB needles were used in the six remaining patients (35.3%); a 22-G needle was mainly used for EUS-TA (82.4%). Of the 17 patients who underwent EUS-TA, three (17.6%) underwent EUS-TA twice because of inconclusive results on the first EUS-TA. In these three patients, the results of the second EUS-TA were used to analyze the ability of EUS-TA for FAIPs.
Using specimens obtained from EUS-TA, histological confirmation of level 1 LPSPs was achieved in 10 patients (58.8%), and a total of 12 patients (70.6%) were histologically diagnosed with level 1 or 2 LPSPs. In other words, the sensitivity of EUS-TA for detecting level 1/2 LPSP was 71%. According to the type of EUS-TA needle, level 1 LPSP was confirmed in five of six patients (83.3%) who underwent EUS-TA using EUS-FNB needles, whereas it was confirmed in five of 11 patients (45.5%) who underwent EUS-TA using conventional EUS-FNA needles. Four patients histologically did not have pancreatic malignancy, and three of them underwent a steroid trial. Only one patient (5.9%) underwent surgery due to a suspected adenocarcinoma, evidence for which was obtained from EUS-TA specimens. No adverse events related to EUS-TA procedures were observed in patients with FAIPs.
Impact of EUS-TA on the confirmation of AIPs for patients with FAIPs
Details of the respective diagnostic criteria used to confirm the presence of AIPs in 23 patients based on the ICDC are shown in Table 4. Of the 17 patients with FAIPs who underwent EUS-TA, 3 (17.6%) were diagnosed with definitive type 1 AIPs using serological, ductal imaging, and OOI listed in the ICDC (level 1 serology plus level 2 ductal imaging, n=2; level 1 serology plus level 1 OOI, n= 1), and 10 (58.8%) were diagnosed with type 1 AIPs based only on the histological results obtained from EUS-TA (level 1 LPSP, Fig. 2). Three patients (17.6%) were scheduled to undergo a steroid trial after obtaining histological findings from EUS-TA specimens (level 2 LPSP, n=1; no malignancy, n=2), followed by a diagnosis of AIP (definitive type 1 AIP, n=1; probable type 1 AIP, n=1; and AIP-NOS, n=1). The remaining patients (5.9%) underwent pancreatic surgery after histological diagnosis using EUS-TA. Therefore, the diagnostic confirmation of AIPs for 13 patients (76.5%) was aided by the results of EUS-TA on pancreatic lesions.
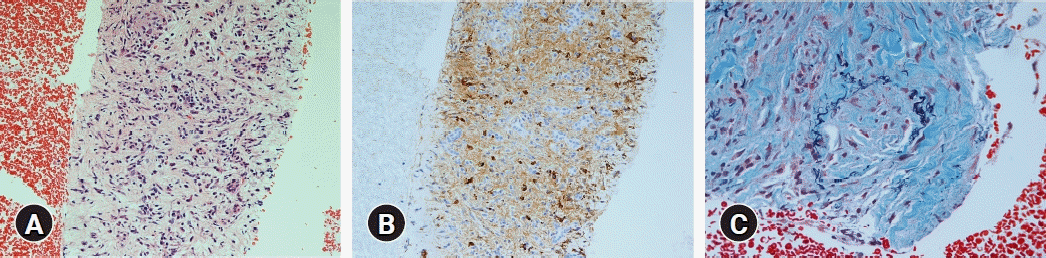
This patient (patient no. 12 in Table 4) was referred to our hospital due to a mass lesion in the pancreatic body. Histological findings of the specimens obtained from the mass lesion by using endoscopic ultrasound-guided fine needle biopsy: The results of hematoxylin and eosin staining for the specimens showed abundant lymphoplasmacytic infiltration and storiform fibrosis (A, ×200). Using IgG4 immunostaining for the specimen, a large number of IgG4-positive plasma cells (B, ×200) was detected. Obliterative phlebitis was detected by using Elastica-Masson staining (C, ×400).
In contrast, three of the six patients who did not undergo EUS-TA (50.0%) underwent surgery for pancreatic lesions because PDACs could not be excluded from the preoperative diagnoses. Therefore, the surgery rate for FAIP lesions was higher for patients who did not undergo EUS-TA than for those who did (50% vs. 6%).
DISCUSSION
This study demonstrated that homogeneous delayed enhancement on CECT was the most useful clinical factor for discriminating FAIPs from PDACs. In other words, when a focal pancreatic lesion involving this enhanced pattern is observed in CECT images, patients should be considered to have possible AIPs. In addition, EUS-TA aided the diagnostic confirmation of AIPs in 76% of the patients with FAIPs, and 50% of those with FAIPs who did not undergo EUS-TA underwent unnecessary surgery for their pancreatic lesions, which indicates that EUS-TA is mandatory for the diagnosis of FAIPs.
Although it is clear that the ICDC has improved the sensitivity for the determination of AIPs,23 surgery has still been undertaken for some patients with FAIPs, even after the proposal of the ICDC.20,21 Based on the ICDC, since FAIPs have high thresholds for their diagnostic confirmation without histological findings, especially when there are no high serum IgG4 levels or OOI, EUS-TA is considered to be key for obtaining accurate diagnoses of FAIPs without clinical findings specific to AIPs. However, since histological diagnosis of AIP using EUS-TA requires an adequate amount of tissues derived from pancreatic lesions, the sensitivity of EUS-FNA for the determination of definitive or probable AIPs is not sufficient (approximately 60%).15-17 Therefore, it is important to recognize the possibility of AIP through clinical findings, excluding the histological ones. Although homogenous delayed enhancement in imaging studies has already been well known as one of the useful findings indicative of AIP, this study showed that this imaging finding was the most promising one for discriminating “FAIP” from PDAC among reported non-histological factors related to AIP. Certainly, this promising and well-known CT finding can lead to EUS-TA with a strong suspicion of AIP.
EUS-TA has recently been emphasized for diagnosing AIPs because of its role as a strong determinant of the confirmation of AIPs based on the ICDC. Based on the results of this study, EUS-TA plays a role in diagnosing FAIPs. Moreover, even when a definitive histological diagnosis cannot be obtained from EUS-TA, if there is a lack of histological findings of malignancy, steroid trials should be performed. In this study, EUS-TA was needed in 76% of patients with FAIPs to confirm AIPs. Therefore, this study clarified that EUS-TA is essential for the diagnosis of FAIPs. In addition, EUS-FNB needles, such as Franseen and fork-tip needles, have recently become available for tissue acquisition from AIP lesions, which have been shown to have a high sensitivity of approximately 80% for assigning the histology as level 1 or 2 LPSP.18,19 On the other hand, EUS-TA using a conventional 22G FNA needle affords a sensitivity of approximately 60% for detecting level 1 or 2 LPSP.15-17 Therefore, it appears that the histological results of EUS-TA in previous reports are relatively consistent with those in this study (detection rates of level 1 LPSP: EUS-FNB needles, 83%; conventional EUS-FNA needles, 45%). In the near future, considering the promising results of EUS-FNB using newly designed needles for AIP lesions, the combination of EUS-FNB and steroid trials for FAIP lesions may become comparable to EUS-FNA for PDAC lesions, the sensitivity of which for detecting malignancy has been reported to be very high (almost 90%).24-26
Our study had several limitations. First, this was a retrospective study with small sample size, conducted at a single institution. Second, for the diagnostic ability of EUS-TA to determine the presence of AIPs, this study provided only the results of sensitivity because patients who were finally found to not have AIPs despite having been suspected of having AIPs were not included in the study. Third, various EUS-TA needles, including EUS-FNB needles, were used at the discretion of the endosonographers. Fourth, since no patients with type 2 FAIPs were included in this study, the clinical implications of EUS-TA in patients with type 2 FAIPs remain unclear. Fifth, to investigate the outcome measurement of 1), only resected PDAC patients in whom serum CA19-9/IgG4 levels were measured and who underwent preoperative ERP were selected as the control PDAC group, whereas some FAIP patients (n=8, 35%) did not undergo ERP for the diagnosis of their pancreatic lesions. This may have caused selection bias in the investigation of comparisons between the two groups.
In conclusion, homogeneous delayed enhancement of CECT is the most useful clinical factor for discriminating FAIPs from PDACs and can lead to an increase in the performance of EUS-TA with the possibility of AIPs in patients with pancreatic focal lesions showing enhanced patterns. EUS-TA is mandatory for the diagnostic confirmation of FAIP lesions and can contribute to a reduction in the rate of unnecessary pancreatic surgeries for patients with FAIPs.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We thank Fumiyoshi Fujishima, MD, PhD, Department of Pathology, Tohoku University School of Medicine and Miwa Uzuki, MD, PhD, Department of Nursing, Faculty of Medical Science and Welfare, Tohoku Bunka Gakuen University for histological evaluations using cytological specimens and resected specimens from all subjects, and all staff in the Department of Pathology at Sendai City Medical Center for immunostaining all specimens in this case. In addition, we are grateful to Dr. Brian Breedlove, Associate Professor, Tohoku University School of Science, for the English proofreading.
Author Contributions
Conceptualization: KY, SK; Date curation: KY, SK; Formal analysis: KY, SK, TT, TSaw, YN, KI; Investigation: KY, SK, YK, TO, HK, TSak, KM, FM, HA, HO, MO; Methodology: KY, SK; Project administration: KY, SK; Supervision: SK, KI; Writing–original draft: KY, SK; Writing–review & editing: all authors.