AbstractBackground/AimsEndoscopic ultrasound gallbladder drainage (EUS-GBD) is gaining attention as a treatment method for cholecystitis. However, only a few studies have assessed the outcomes of permanent stenting with EUS-GBD. Therefore, we evaluated the clinical outcomes of permanent stenting using EUS-GBD.
MethodsThis was a retrospective, single-center cohort study. The criteria for EUS-GBD at our institution are a high risk for surgery, inability to perform surgery owing to poor performance status, and inability to obtain consent for emergency surgery. EUS-GBD was performed using a 7-Fr double-pigtail plastic stent with a dilating device. The primary outcomes were the recurrence-free rate of cholecystitis and the late-stage complication-avoidance rate. Secondary outcomes were technical success, clinical success, and procedural adverse events.
ResultsA total of 41 patients were included in the analysis. The median follow-up period was 168 (range, 10â1,238) days. The recurrence-free and late-stage complication-avoidance rates during the follow-up period were 95% (38 cases) and 90% (36 cases), respectively. There were only two cases of cholecystitis recurrence during the study period.
INTRODUCTIONAcute cholecystitis is commonly encountered in clinical practice, and laparoscopic cholecystectomy (LC) is the standard treatment method. In cases resistant to acute cholecystitis, early stage surgery is recommended, regardless of the elapsed time since onset.1
Gallbladder drainage is required when conservative therapy fails in patients with advanced underlying malignancies, multiple comorbidities, or those unsuitable for surgery owing to old age. Percutaneous transhepatic gallbladder drainage (PTGBD) is the standard treatment for these cases,2 but drainage-related complications are occasionally observed. For example, the fistula may be dislodged when it is not formed because of difficulty in drain management because of aging or dementia, or the drainage tube cannot be removed due to cystic duct obstruction.
The Tokyo Guidelines 2018 state that facilities with therapeutic endoscopy specialists may consider transpapillary or endoscopic ultrasound (EUS) drainage.1 Endoscopic gallbladder drainage, such as endoscopic gallbladder stenting (EGBS) and EUS gallbladder drainage (EUS-GBD), as an alternative method that enables long-term internal drainage, avoids percutaneous drainage-related complications, and reduces recurrent gallbladder inflammation.
The utility of EUS-GBD has recently attracted attention for cases in which cholecystectomy risks are high and tube management cannot be conducted. The history of EUS-GBD was first reported in 1996 by Wiersema et al.3 on EUS-guided cholangiopancreatography. Through various innovations, such as EUS-guided bilioduodenal anastomosis,4 which forms a fistula between the common bile duct and duodenum, this method has been increasingly used in medical treatment. The series of EUS-GBD procedures involves observation of the gallbladder in a transgastric or transduodenal manner under EUS guidance and placement of a guidewire in the gallbladder after puncturing with a needle while confirming that there are no intervening blood vessels. Then, the puncture route is expanded with a mechanical dilator, an electrocautery dilator, or an approximate balloon dilator for fistula dilation. Finally, a naso-gallbladder drainage tube, double-pigtail plastic stent (DPPS), or self-expandable metallic stent (SEMS) is positioned to allow gallbladder drainage. According to one study, for general EUS-GBD procedures, the success rate was 98.0%, treatment response rate was 94.4%, and accident occurrence rate was 12.1%.5
Research on EUS-GBD is limited, with several studies including low case numbers or short-term performance; however, only a few studies assessing the performance outcomes of a large number of cases have been published.5,6 Therefore, we conducted a survey at our hospital to analyze the outcomes of EUS-GBD cases with permanent endoscopic fistulae for non-surgical purposes. In addition, studies on SEMS have been published,5,7 but studies using plastic stents (PSs) are limited.8,9 PSs are considered sufficient because they are unlikely to deviate from the gallbladder, they can be removed, and food residues cannot enter the gallbladder through them. In addition, they are less expensive than metallic stents (MSs). Furthermore, in our experience, we encountered few complications, and there appeared to be no difference in the clinical success rates compared with those in previous reports.4,8-10 Therefore, we performed EUS-GBD using PSs.
In this study, we aimed to evaluate the performance of EUS-GBD in cases where surgery was not possible (excluding bridge-to-surgery [BTS] cases) to investigate the efficacy and safety of EUS-GBD using DPPSs.
METHODSPatientsThe study was conducted during the period from June 2016 to November 2020. The study participants were patients with acute cholecystitis who were admitted to the NTT Tokyo Medical Center in Tokyo. Patients with permanent stent placement were eligible for this study if they did not require emergency cholecystectomy or BTS (Fig. 1).
The treatment of acute cholecystitis at our hospital follows the guidelines for its diagnosis and treatment.1 For inoperable cases, we chose EUS-GBD, PTGBD, or EGBS when drainage was required. In the case of EGBS, it is not always possible to complete the procedure, such as when the papillary approach is difficult or when the cystic duct is perforated when approaching the gallbladder through the duct. In such cases, other approaches should be considered, and EUS-GBD should be performed if possible.
Indications for EUS-GBD at our hospital include operation-resistant cases that are at high surgical risk and exhibit poor performance status response, cases where consent for emergency surgery cannot be obtained, and cases of internal fistula formation in PTGBD (a patient who has a PTGBD in place but needs EUS-GBD is a patient whose cholecystitis recurs when the PTGBD is clamped). In addition, cases with ineffective antibiotic treatment are recommended for drainage. Patients with poor respiratory conditions due to sedation during the procedure, those with gallbladder perforation/necrotizing cholecystitis, and those receiving oral antithrombotic drugs were excluded. Patients not clearly diagnosed with cholecystitis were excluded if there was a possibility of BTS; however, the study also excluded patients undergoing BTS.
ProceduresThe equipment used when performing EUS-GBD included an echoendoscope (GF-UCT260; Olympus Medical Systems), an ultrasonic endoscopic video processor (EU-ME1/2; Olympus Medical Systems), a 19-G needle (EZ-Shot3; Olympus Medical Systems), a 0.025-inch guidewire (VisiGlide2; Olympus Medical Systems), a 4-mm balloon dilator (REN biliary balloon catheter; Kaneka Medix), an ultra-tapered mechanical dilator (ES Dilator; Zeon Medical), and a 7-Fr, 10-cm (Through & Pass; Gadelius Medical) or 5-Fr endoscopic nasal biliary drainage tube (SilkyPass; Boston Scientific Japan) DPPS.
The patients were sedated in the prone position using midazolam or pentazocine. CO2 insufflation was used to prevent abdominal compartment syndrome associated with pneumoperitoneum due to intraoperative perforation.
The gallbladder was visualized by ultrasonography from either the duodenal bulb or antrum of the stomach. The duodenal bulb, which is closer to the gallbladder than to the antrum of the stomach, is often selected as the puncture site to reduce bile leakage and minimize stent movement (Fig. 2). A 19-G needle was used to puncture the gallbladder after evaluating the void of intervening blood vessels in Doppler mode, and 20 mL of bile was aspirated. The aspirated bile was submitted for culture, after which contrast-enhanced evaluations were conducted.
A 0.025-inch guidewire was inserted into the bile sac cavity and wound into the coil, after which a 4-mm balloon dilator was used to expand the fistula. After expansion, a 7-Fr DPPS was placed between the gallbladder and duodenal bulb or antrum of the stomach at a length of 10 cm (Fig. 2). Whether dilators are used to create a wider fistula tract depends on the individual endoscopist.
Follow-upThe follow-up period for all patients covered the period from the date of treatment to either the date of death or the final outpatient visit within the survey period.
Simple abdominal radiography and blood tests were performed the day after treatment in all patients. Oral intake was started at the time point when improvements in clinical symptoms or clinical findings were confirmed.
BTS may occur after acute cholecystitis has subsided, but such cases were excluded from this study. Thus, DPPSs were left without replacement during the follow-up period.
Outcome variablesThe primary outcomes were the recurrence-free rate of cholecystitis and the late-stage complication-avoidance rate. The recurrence-free rate of cholecystitis was defined as the rate at which cholecystitis improved after DPPS placement with no recurrence. The late-stage complication-avoidance rate was defined as the fraction of patients who did not exhibit late-stage complications such as adverse events (AEs), cholecystitis recurrence, stent deviation, delayed-onset bleeding, or delayed-onset perforation at more than 2 weeks post-treatment.
The secondary outcomes were technical and clinical success, procedural AEs, and procedure time. Technical success was defined as the fraction of cases in which the stipulated procedure or treatment was implemented without any problems. Clinical success was defined as the proportion of cases in which a therapeutic effect was achieved.
Therapeutic effect was defined as at least two of the following three findings normalizing within 72 hours: leukocytosis, elevated body temperature, and abdominal pain.
Procedural AEs included puncture bleeding, bile leakage, peritonitis, and pneumoperitoneum. AEs were generally described according to the American Society for Gastrointestinal Endoscopy lexicon.10
Cholecystitis ârelapseâ was defined as cholecystitis worsening before it was completely cured. Cholecystitis ârecurrenceâ was defined as cholecystitis reoccurring after it was thought to be cured.
Statistical analysisDescriptive continuous variables are presented as numbers (percentages), medians (ranges), means (ranges), or standard deviations. The Kaplan-Meier analysis of recurrence-free and late-stage complication rates was performed. Statistical analysis was performed using EZR software ver. 1.54, 2020 (The R Foundation for Statistical Computing).
Ethical statementsThe research protocol was approved by the Institutional Review Board of NTT Tokyo Medical Center Tokyo (Research registration no. 18-313), and the study was conducted in accordance with the provisions of the Declaration of Helsinki. Informed consent was obtained from all the patients.
RESULTSPatient characteristicsSeventy-three patients underwent EUS-GBD at our hospital from June 2016 to November 2020. Among these cases, 32 in which BTS was possible were excluded. In total, 41 cases met the inclusion criteria, patient consent was obtained, and permanent stent placement was performed (Fig. 1, Table 1). The median age was 80.2Âą9.9 (range, 48â98) years, and 26 patients were male.
Gallstones (25 cases) were the most common cause of cholecystitis. Other causes included metal stents (10 cases), previous PS (one case), cancer (four cases), and ischemia (one case). Cholecystitis was severe in five patients and moderate in 36 patients. Except for one severe case in which EGBS was difficult and the patient underwent EUS-GBD, the procedure began in all cases with EUS-GBD as the objective. One case in which EGBS was planned was changed to EUS-GBD during the procedure because of the difficulty of the transpapillary approach. The median follow-up period was 168 (range, 10â1,238) days.
One case was considered a clinical failure because the patient relapsed due to hemorrhage after EUS-GBD.
Primary outcomesThe recurrence-free and late-stage complication-avoidance rates during the follow-up period were 95% (38 cases) and 90% (36 cases), respectively (Table 2, Fig. 3). Among the recurrent cases, one experienced stent deviation because of scope interference while undergoing endoscopic retrograde cholangiopancreatography 2 months after treatment, with cholecystitis recurrence occurring a few days after endoscopic retrograde cholangiopancreatography. In the other recurrent case, a 5-Fr endoscopic nasal biliary drainage tube was used first because 7-Fr stent placement was difficult. After the stent was cut for internal fistula formation, recurrence was observed for 12 days. EUS-GBD was repeated in both recurrent cases, after which no subsequent recurrence was observed.
Other late-stage complications included two cases (4.9%) of stent migration; however, there was no recurrence of cholecystitis (Fig. 3, Table 2).
In Figure 3, the recurrence-free rate of cholecystitis and late-stage complication-avoidance rate are shown on the vertical axis using the Kaplan-Meier method, and the number of follow-up days on the horizontal axis.
Early adverse eventsComplications occurred within 2 weeks of treatment in 10 (24.4%) cases. Among these cases, there were 9 (22.0%) cases of biliary peritonitis and 1 (2.4%) case of bleeding. No intraperitoneal abscesses or perforations were observed. Stent obstruction occurred as a result of bleeding, resulting in relapse in one case (Table 3). This was defined as treatment failure because the stent obstruction that occurred before cholecystitis resolved.
The symptoms related to bile leakage and mild peritonitis were mainly abdominal pain after treatment, which was treated conservatively and improved with antibiotics and fasting.
Clinical course and long-term prognosisA positive clinical response was observed after EUS-GBD in 40 cases without the need for additional transdermal treatment. Cholecystitis recurrence was observed in only two cases during the follow-up period.
Figure 4 shows a laparoscopic image of a patient who underwent LC after EUS-GBD at our hospital more than 1 month after the procedure. In this image, the fistula between the duodenum and the gallbladder is firmly established after EUS-GBD.
Analysis to identify risk factors for acute cholecystitis recurrent-free and adverse eventsWe performed a multivariate analysis to identify the risk factors for recurrence-free acute cholecystitis and AEs. However, no significant difference was observed. The examined factors included age, sex, cause of cholecystitis, severity of cholecystitis, puncture site, use of ES dilators, and procedure time.
Endosonographic examination of the gallbladderWe examined endosonographic findings of the gallbladder. The findings were âgallbladder enlargementâ in 51.2%, âthickening of the gallbladder wallâ in 51.2%, âdebris echoâ in 85.4%, and âhypoechoic layer of the gallbladder wallâ in 17.1% of the cases. âPericholecystic fluidâ and âgas imagingâ were noted in 0% of cases.
DISCUSSIONIn this study, we evaluated the long-term performance of EUS-GBD in cases where surgery was not possible to investigate the efficacy and safety of EUS-GBD using DPPSs. Permanent stent placement by EUS-GBD, which used DPPS for inoperable acute cholecystitis, exhibited a high cholecystitis recurrence-free rate (95%) and late-stage complication-avoidance rate (90%), making it an effective method that results in long-term therapeutic effects. The technical and clinical success rates were 100% and 97.6%, respectively.
DPPSs have a small diameter and cannot be secured to the duodenal or gastric lumen; therefore, there are concerns regarding insufficient drainage and bile leakage when DPPSs are used. However, in the present study, we believe that the high recurrence-free rate was attributed to the fact that even if there was obstruction of the DPPS, a fistula would form between the gallbladder and the duodenum or stomach, resulting in constant drainage along the gap, thus preventing recurrence. This is similar to the mechanism by which a fistula is formed after PTGBD treatment, in which bile leakage occurs between gaps.
In the present case, fistula formation was firmly established and confirmed more than 1 month after the operation, depending on the BTS case (Fig. 4). On observing the EUS-GBD puncture site in cases where a common bile duct stone removal treatment was necessary after permanent stent placement, even if the DPPS moved slightly in and out of the puncture site, drainage would occur alongside the DPPS as long as a fistula had formed.
To date, there have been studies on SEMS, lumen-apposing metal stents, and nasocystic tubes,5,7,8 but DPPSs have been used for EUS-GBD at our center. One reason for this choice is that DPPSs are cheaper than MSs. MS placement also has an increased risk of pneumoperitoneum and duodenal perforation, and MS use could also result in stent obstruction and stent migration.11 As SEMSs are self-expandable, cholecystitis recurrence may become more likely than PS placement because the gap around the fistula does not form as easily in the event of an obstruction. After the treatment of cholecystitis with PTGBD, the tube may be self-removed before fistula formation. In addition, continued percutaneous exposure of the tube leads to a decreased quality of life, and skin problems due to side leakage of bile from the fistula may persist while the tube is in place.
Many patients are unsuitable surgical candidates because of old age or various underlying diseases. In these cases, PTGBD is considered the standard drainage method,2 but this method does not address the problems of drainage-related accidents and drainage tube removal. In such cases, permanent stent placement using EUS-GBD may be a valid alternative.
In a previous study, the incidence rate of treatment-induced complications was approximately 12.1%, although there were some differences between MS and PS.6 Other AEs were reported in 9.9% of lumen-apposing MS, 12.3% of SEMS, and 18.2% of PS cases. In comparison, 7.3% of the AEs in this study were considered relatively low, and 4.9% were mild peritonitis, which improved with conservative treatment and was not related to the patientâs condition. This contributed little to prognosis. In this study, compared with a complication rate of 24.4% among 73 patients, including BTS cases at our hospital, 7.3% of cases of permanent stenting resulted in a lower complication rate. Peritonitis was thought to be caused by leakage during puncture, which occurred in a certain percentage of cases. In addition, the risk of perforation is much lower with PSs than with MSs, even if the stent is permanently implanted and deviates from its original position.
Based on these considerations, the results of this study demonstrate that permanent stent placement using EUS-GBD for cholecystitis is a safe and effective treatment method. Additionally, EUS-GBD is not a transpapillary approach; thus, there is no risk of pancreatitis complications following endoscopic treatment based on the characteristics of the procedure. To date, no cases of pancreatitis have been reported in previous studies of EUS-GBD.12
This study has some limitations. First, this was a single-center retrospective study with no control group. EUS-GBD is a treatment for which no standard procedures have been established, and the technical ability and judgment of the endoscopic surgeon may play a large role in how the procedure is performed. Furthermore, although the use of lumen-apposing MS is increasing in Japan, the use of MSs is not covered by insurance in EUS-GBD. Therefore, PSs were used in this study. The usefulness of MSs should be examined in future studies.
In conclusion, EUS-GBD using DPPS resulted in a high late-stage complication-avoidance rate, even in the long term, among patients with acute cholecystitis where emergency cholecystectomy was not suitable and permanent stent placement was necessary. Our results indicate that this is a safe and feasible alternative to other drainage methods. Therefore, we hope that the number of patients who undergo EUS-GBD will increase across multiple facilities and that the procedure will be generalized in the future.
NOTESAuthor Contributions
Conceptualization: ES; Data curation: ES; Formal analysis: ES; Funding acquisition: ES, TT, AN, NM; Investigation: ES, YK, ST, YF; Methodology: ES; Project administration: ES; Resource: ES; Software: ES; Supervision: YF, KH; Validation: YF, KH; Visualization: ES; Writingâoriginal draft: ES; Writingâreview & editing: all authors.
Fig. 1.Among the 73 patients with cholecystitis, 32 patients undergoing BTS were excluded, and 41 patients who met the indication criteria and provided consent underwent permanent stenting. BTS, bridge-to-surgery. 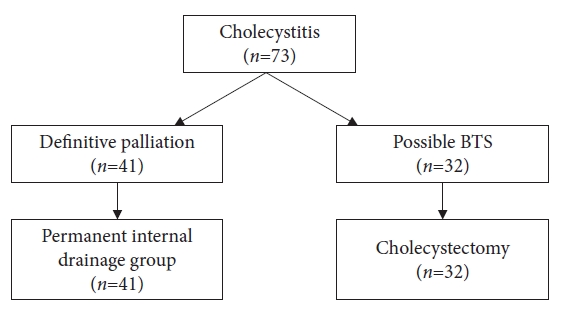
Fig. 2.. Endoscopic ultrasound gallbladder drainage procedure images. (A) Endoscopic ultrasound image during gallbladder puncture. (B) Fluoroscopic image of a balloon catheter being dilated. (C) Fluoroscopic image of plastic stent implantation. (D) Endoscopic image of plastic stent implantation. 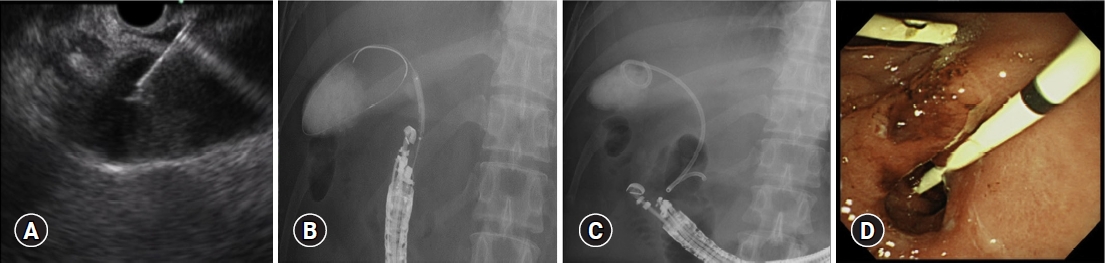
Fig. 3.The horizontal axis presents the number of follow-up days, and the vertical axes present (A) the non-occurrence rate of cholecystitis and (B) the late-stage complication-avoidance rate. 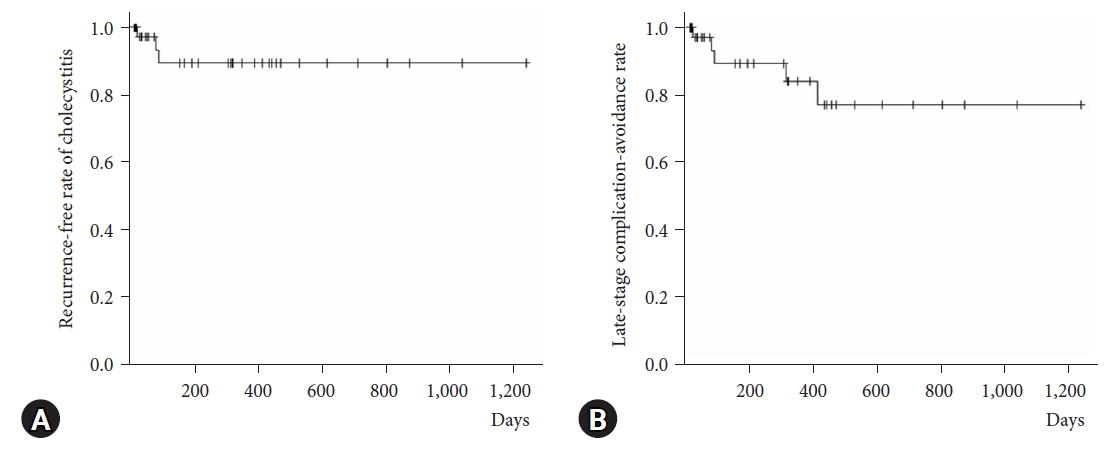
Fig. 4.The fistula between the gallbladder (GB) and the duodenum (DU) is shown by the arrow in the laparoscopic cholecystectomy. 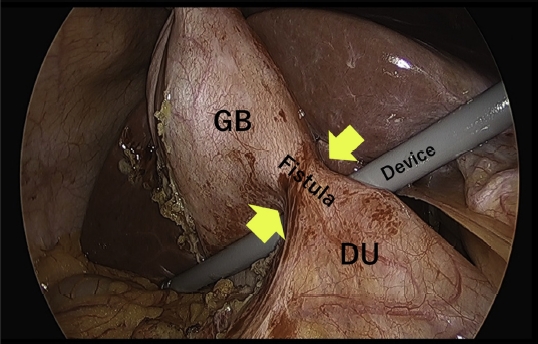
Table 1.Baseline characteristics of patients Table 2.Clinical outcomes after endoscopic ultrasound-guided gallbladder drainage Table 3.Details of endoscopic ultrasound-guided gallbladder drainage procedures REFERENCES1. Takada T. Tokyo Guidelines 2018: updated Tokyo Guidelines for the management of acute cholangitis/acute cholecystitis. J Hepatobiliary Pancreat Sci 2018;25:1â2.
2. Melin MM, Sarr MG, Bender CE, et al. Percutaneous cholecystostomy: a valuable technique in high-risk patients with presumed acute cholecystitis. Br J Surg 1995;82:1274â1277.
3. Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43(2 Pt 1):102â106.
4. Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898â900.
5. Anderloni A, Buda A, Vieceli F, et al. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: a systematic review and pooled analysis. Surg Endosc 2016;30:5200â5208.
6. Matsubara S, Isayama H, Nakai Y, et al. Endoscopic ultrasound-guided gallbladder drainage with a combined internal and external drainage tubes for acute cholecystitis. J Gastroenterol Hepatol 2020;35:1821â1827.
7. Ryozawa S, Fujita N, Irisawa A, et al. Current status of interventional endoscopic ultrasound. Dig Endosc 2017;29:559â566.
8. Dhir V, Isayama H, Itoi T, et al. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig Endosc 2017;29:472â485.
9. Lee SS, Park DH, Hwang CY, et al. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc 2007;66:1008â1012.
10. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446â454.
|
|
|||||||||||||||||||||||||||||||||||||
