Refractory benign biliary stricture due to chronic pancreatitis in two patients treated using endoscopic ultrasound-guided choledochoduodenostomy fistula creation: case reports
Article information
Abstract
Benign biliary stricture (BBS) is a complication of chronic pancreatitis (CP). Despite endoscopic biliary stenting, some patients do not respond to treatment, and they experience recurrent cholangitis. We report two cases of CP with refractory BBS treated using endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) fistula creation. A 50-year-old woman and a 60-year-old man both presented with obstructive jaundice secondary to BBS due to alcoholic CP. They underwent repeated placement of a fully covered self-expandable metal stent for biliary strictures. However, the strictures persisted, causing repeated episodes of cholangitis. Therefore, an EUS-CDS was performed. The stents were eventually removed and the patients became stent-free. These fistulas have remained patent without cholangitis for more than 2.5 years. Fistula creation using EUS-CDS is an effective treatment option for BBS.
INTRODUCTION
Benign biliary stricture (BBS) is a complication of chronic pancreatitis (CP). Previous studies have shown that the incidence of BBS in CP ranges between 3% to 46%, and the strictures are due to extrinsic compression from fibrotic pancreatic tissue in the bile ducts.1,2 The European Society of Gastrointestinal Endoscopy guidelines recommend endoscopic treatment (ET) for CP-related BBS with symptoms of biliary obstruction persisting for >1 month. The guidelines suggest temporary insertion of multiple plastic stents (MPS) or fully covered self-expandable metal stents (FCSEMS).3
Although the efficacy of temporary insertion of an MPS or FCSEMS has been reported,4 some patients do not respond to it. Herein, we report two cases of refractory BBS secondary to CP treated using endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) fistula creation, both of whom were followed-up for a long term.
CASE REPORT
Case 1
A 50-year-old woman presented to our hospital with obstructive jaundice. Endoscopic retrograde cholangiopancreatography (ERCP) revealed intrapancreatic bile duct stricture. The patient underwent endoscopic sphincterotomy and FCSEMS placement (6 mm×6 cm, Niti-S Biliary S-type Stent; TaeWoong Medical). Malignancy was ruled out using biliary cytology and EUS-guided fine-needle aspiration (EUS-FNA). Temporary FCSEMS placement had been performed four times within the two preceding years, following the initial FCSEMS implantation. Finally, a 12-mm-diameter FCSEMS (12×60 mm, Niti-S biliary stent SUPREMO; TaeWoong Medical) was placed to dilate the stricture. Despite these procedures, the stricture persisted and caused recurrent cholangitis. Therefore, EUS-CDS was performed. After removing the FCSEMS placed across the papilla, an echoendoscope (TGF-UC260J; Olympus Medical Systems) was inserted into the duodenal bulb to visualize the common bile duct, which was punctured using a 19-gauge EUS-FNA needle (SonoTip Pro Control; Medico Hirata). After bile aspiration, a 0.025-inch guidewire (Visiglide2; Olympus Medical Systems) was inserted. Fistula dilation was performed using a 6-Fr cautery dilator (Cysto-Gastro-Set; Endo-Flex). Finally, an FCSEMS (10×60 mm, X-suit NIR; Olympus Medical Systems) was inserted along the guidewire and placed through the choledochoduodenal fistula under fluoroscopic and ultrasound-endoscopic imaging. After stent placement, the direction of the distal side of the stent was changed from the oral to the anal side using an endoscope and guidewire, and the procedure was completed (Fig. 1). The following day, no complications were confirmed during computed tomography. While allowing mature fistula creation before removing the stent permanently, scheduled stent exchange was performed at three-month intervals. The 10-mm-diameter FCSEMS used during the EUS-CDS was replaced with a 12-mm-diameter FCSEMS at the first replacement. Finally, we removed the stent 12 months after the EUS-CDS. A scheduled annual follow-up with upper gastrointestinal endoscopy was performed to confirm the condition of the fistula, which has remained patent without developing cholangitis for the past 3.5 years (Fig. 2). Fistula dilation was not performed during the treatment.

Endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) procedure. (A) Fluoroscopic image of a fully covered self-expandable metal stent being placed during EUS-CDS. (B) Endoscopic image of an inserted fully covered self-expandable metal stent using EUS-CDS.
Case 2
A 60-year-old man with a main pancreatic duct stricture and intrapancreatic bile duct was referred to our hospital. Malignancy was ruled out using EUS-FNA of the pancreatic head and pancreatic juice cytology. The patient was diagnosed with BBS secondary to alcoholic CP and obstructive jaundice. Thus, the patient underwent temporary FCSEMS (8 mm×6 cm, Niti-S Biliary S-type Stent) placement in the stricture for four months, after which the stent was removed. However, the stricture persisted, causing recurrent episodes of cholangitis. Therefore, EUS-CDS with a 12-mm FCSEMS (12×50 mm, BONASTENT M-Intraductal; Sci-Tech Inc.) was performed one month after the FCSEMS removal (Fig. 3). The stent was dislocated six months after the EUS-CDS. We performed fistula dilation with a balloon up to 10 mm in diameter (CRE PRO wire-guided biliary dilation balloon catheter; Boston Scientific) without stent placement (Fig. 4). After 26 months, the patient developed stone-induced cholangitis. Fistula dilation was performed with a balloon up to 13.5 mm in diameter (CRE PRO wire-guided biliary dilation balloon catheter) for stone treatment. Four clips (Sure clip; Micro-Tech) were then placed around the fistula to maintain fistula patency (Fig. 5). No complications were observed during the treatment. The fistula has remained patent for five years after the EUS-CDS, and the patient did not develop any abdominal pain or cholangitis for 2.5 years after the last fistula dilation (Fig. 5).
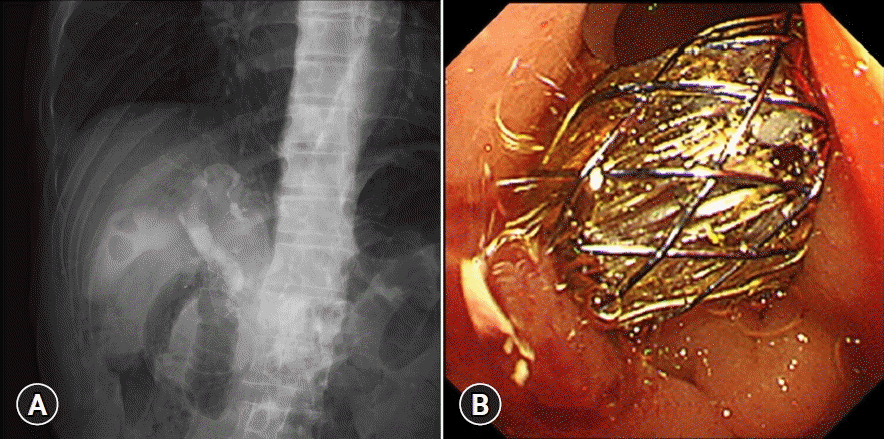
Endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) procedure. (A) Fluoroscopic image of a fully covered self-expandable metal stent being placed during EUS-CDS. (B) Endoscopic image of an inserted fully covered self-expandable metal stent using EUS-CDS.
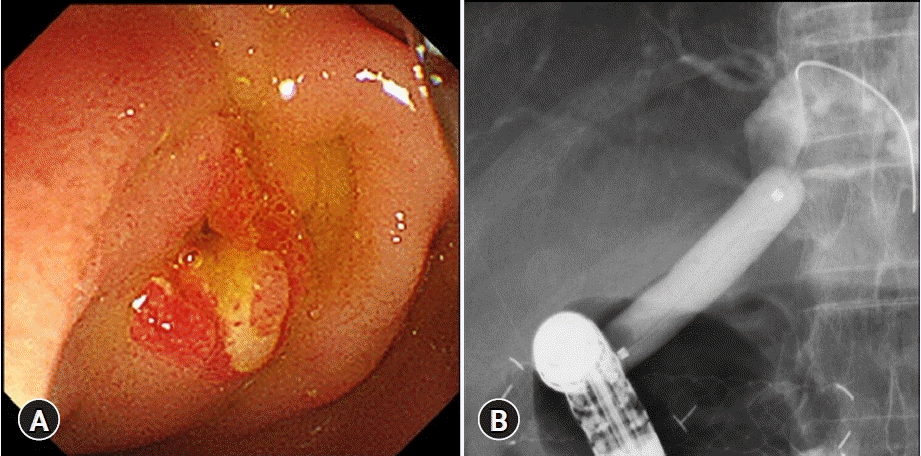
Findings at the time of fistula dilation at six months after endoscopic ultrasound-guided choledochoduodenostomy. (A) At the time of stent dislocation. (B) Fistula dilation with a balloon up to 10-mm diameter.

Fistula treatment at 32 months after endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) and subsequent endoscopic findings. (A) Fistula before balloon dilation. (B) Fistula dilation with a balloon up to 13.5 mm diameter. (C) Fistula after the balloon dilation. (D) Clips placed around the fistula. (E) Patent fistula at two years after the balloon dilation and five years from the EUS-CDS.
DISCUSSION
BBS occurs from chronic inflammatory pancreaticobiliary pathologies, postoperative bile duct injury, or biliary anastomoses following liver transplantation. CP is the most common nonsurgical cause of BBS, especially in the intrapancreatic bile duct.5 The European Society of Gastrointestinal Endoscopy guidelines recommend ET for symptoms of biliary obstruction that persist for >1 month, with temporary insertion of an MPS or FCSEMS. Surgery should be considered one year after an unsuccessful ET.3 A previous study reported stricture resolution rates of 77.1% and 75.8%, and the mean number of ERCP of 3.9±1.3 vs. 2.6±1.3 for MPS and FCSEMS, respectively, at the 24-month follow-up.4 A problem with ET for BBS secondary to CP is that CP is a lifelong disease that may eventually lead to stricture recurrence. Lakhtakia S et al. reported that a five-year follow-up study of temporary implantation of an FCSEMS for CP showed a stricture resolution rate of 62%.6
EUS-CDS is a transluminal technique that creates a fistula connecting the duodenum and dilated common biliary duct.7 It is commonly used in patients with distal malignant biliary obstruction following a failed ERCP.8 Moreover, it is useful in patients undergoing primary biliary drainage.9,10 Concerning the safety of EUS-CDS, previous reports have reported technical and clinical success rates of 93.67% and 91.23%, respectively.11 A previous randomized controlled trial comparing EUS-CDS and ERCP reported a lower incidence of adverse events (AEs) in a EUS-CDS group (6.3%) than in an ERCP group (19.7%, p=0.03),12 suggesting that the procedure can be performed safely. A recent study has shown that using an FCSEMS is reasonable for preventing AEs to improve safety.13 Herein, we performed EUS-CDS fistula creation for refractory BBS in the two cases. Fistula creation was performed using EUS-CDS in case 1, and stent exchange was scheduled for 12 months. The fistula has remained patent without cholangitis for 3.5 years after stent removal. Fistula creation was performed using EUS-CDS, balloon dilation, and clipping around the fistula in case 2; the fistula has remained patent for five years after the EUS-CDS. Additional treatment was not performed during the 3.5-year follow-up period after attaining a stent-free status in case 1. Balloon dilation was performed only once during the 4.5 years after attaining a stent-free status in case 2. Our findings suggest that EUS-CDS fistula creation may reduce the number of procedures required for refractory BBS compared with past strategies. Although the frequency of the procedures was similar, EUS-CDS would be preferable if stents were not required. Few studies have reported EUS-CDS fistula creation with BBS. In a previous case report, a fistula was formed by EUS-CDS using a 10-mm FCSEMS for refractory BBS secondary to CP. The stent was removed six months after the EUS-CDS. The patient reported no fever or abdominal pain for >8 months after the stent removal.14 Unlike previous reports, we performed balloon dilation using a large-diameter stent. We also placed clips around the CDS fistula to maintain it. However, the clips may not be involved in long-term patency because they spontaneously dislocated during the treatment course. Additionally, this stent-free fistula creation method using EUS-CDS may be useful in treating malignant biliary strictures. In a previous report, a patient with malignant biliary obstruction due to pancreatic cancer underwent EUS-CDS using an FCSEMS. The stent was removed 10 months after the EUS-CDS and remained asymptomatically patent for 15 months.15 Complications arising from prolonged stenting in EUS-CDS may not occur in a stent-free environment. The EUS-CDS may result in stent-free fistula creation for biliary strictures, which we call “neo-papilla.” However, a method for creating a permanent fistula remains unknown. Further aspects of this treatment, such as duration of stent placement, stent exchange schedule, necessity of clip placement, balloon dilation, and indications need to be investigated to better understand how to create a neo-papilla. In conclusion, EUS-CDS fistula creation may be a useful treatment option for refractory BBS.
Notes
Conflicts of Interest
Dr. Nobumasa Mizuno reports having received grants and personal fees from Yakult Honsha, grants and personal fees from AstraZeneca, grants and personal fees from Novartis, grants and personal fees from MSD, grants from Ono Pharmaceutical, grants from Taiho Pharmaceutical, and grants from Eisai outside the submitted work. The other authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: SI, NO, KH; Data curation: SI; Investigation: SI; Methodology: KH; Project administration: KH; Resources: SI, NO; Supervision: KH; Validation: NM, SH, TK, YK, TY; Visualization: SI; Writing–original draft: SI; Writing–review & editing: all authors.

