INTRODUCTION
In recent years, the detection rate of duodenal tumors has increased with advances in endoscopic equipment.1,2 Thus, endoscopic treatment for superficial non-ampullary duodenal tumors (SNADETs) is being performed with increasing frequency.1,3 Although endoscopic submucosal dissection (ESD) for SNADETs has been associated with a high rate of en bloc resection and a low possibility of recurrence, the technique for ESD remains challenging.4 Duodenal mucosa is generally thinner compared to mucosa from other parts of the gastrointestinal tract, which leads to invisibility of the submucosal layer during ESD. Consequently, the incidence of intraoperative perforation is extremely high during duodenal ESD than during ESD in other parts of the gastrointestinal tract.5-7 Therefore, novel methods for making duodenal ESD simple and safe are required.
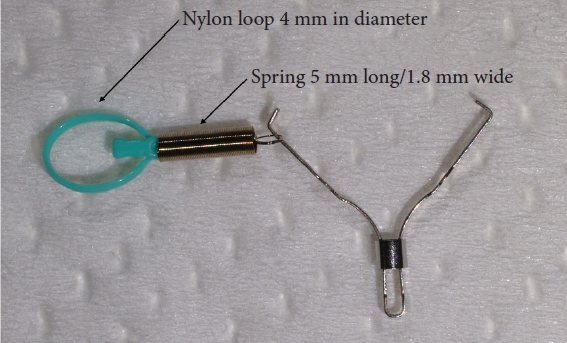
The S-O clip (ZEON Medical) was launched in 2018. It is a traction device developed primarily for colorectal ESD (Fig. 1).8 It is comprised of a 5-mm long and 1.8-mm wide spring, with a 4-mm nylon loop at the clip claw. It allows a stable and clear view of the submucosal layer during dissection after circumferential incision, making colonic ESD safer and faster. The utility of S-O clips in colonic and gastric ESD has already been evaluated.8,9 However, no studies have discussed the usefulness of S-O clips in duodenal ESD.
Thus, this study aimed to assess the usefulness and safety of duodenal ESD using an S-O clip for SNADETs.
METHODS
Study design and patient recruitment
This retrospective observational study was conducted at Sendai Kousei Hospital, a tertiary referral center in Japan. All consecutive patients who underwent ESD for SNADETs at our institution between January 2011 and December 2021 were included.
The inclusion criteria were SNADETs sized >10 mm that had been pathologically diagnosed as adenomas, intramucosal adenocarcinomas, or submucosal adenocarcinomas. The exclusion criteria were SNADETs with endoscopic signs of deep submucosal invasion or SNADETs that had spread to the ampulla of Vater.
Patients who underwent duodenal ESD with an S-O clip (S-O group) were compared with patients who underwent conventional ESD (control group).
ESD
All ESD procedures were performed using a single-channel endoscope (GIF-H260J; Olympus Corp.) with a transparent hood (D-201-11804; Olympus Medical Systems) under intravenous anesthesia with propofol and pentazocine. We used the VIO300D system (Erbe Elektromedizin GmbH) as electrosurgical generator.
In the control group, we followed a conventional ESD strategy for SNADETs. Initially, a mucosal incision was initiated from the oral side of the lesion using a hook knife (KD-625LR; Olympus Medical Systems) after injection of sodium hyaluronate (MucoUp; Boston Scientific). After creating a mucosal flap for submucosal placement of the endoscope, a circumferential incision was made. Finally, the submucosal layer was dissected from the oral to the anal side.
In the S-O group, the procedure was different from that used for conventional ESD (Fig. 2). A circumferential incision was made without creating a mucosal flap. An S-O clip was then attached to the edge of the oral side of the resected mucosa. Another normal clip was used to hold the loop on the S-O clip and was pulled to the proximal duodenal wall opposite to the lesion. This process resulted in clear visualization of the submucosa. After completing the submucosal dissection, the nylon loop on the S-O clip was cut off using a hook knife. Although we have previously reported this procedure,10 Supplementary Video 1 in this article also represent how to perform duodenal ESD using S-O clip.10
Expert endoscopists who had performed over 1,000 ESD procedures for esophageal, gastric, and colorectal neoplasms performed the ESD procedures in the present study.
Pathological diagnoses from resected specimens were performed by a highly experienced clinical pathologist.
Management of mucosal defects after ESD
Post-ESD ulcers were closed using simple clips. If an ulcer was difficult to close, a closure method using an endoscopic detachable snare and clips was utilized.11 Otherwise, difficult-to-close wounds were covered with polyglycolic acid sheets and fibrin glue.12 Additionally, when a wound was in the descending part of colon and difficult to close, an endoscopic nasal biliary and pancreatic drainage (ENPBD) tube was used, since biliary/pancreatic juices may have harmful effects on mucosal defects, leading to a delayed perforation.13
Management after ESD
In patients who underwent duodenal ESD using the closing method, a second endoscopy was performed to check for any delayed perforation or bleeding on postoperative day (POD) 1. Patients were instructed to avoid eating and were hydrated intravenously for 1 day after the surgery. If there were no signs of postoperative perforation or bleeding, patients started eating on POD 2 and were discharged on POD 7.
A second endoscopy was performed on POD 3 in patients who underwent wound management with sheet coverage and/or ENPBD. If there were no signs of delayed perforation or bleeding, the tube was removed. Patients were instructed to avoid eating and were hydrated intravenously for 3 days. After the second endoscopy, they received liquid food on POD 4 and were discharged on POD 9. In case of delayed complications, computed tomography and blood tests were performed.
Outcomes
Intraoperative perforation rate was the primary outcome of this study. Procedure time and R0 resection rate were the secondary outcomes.
Definitions
Procedure time was defined as the time from the first local injection to lesion removal. Intraoperative perforation was defined as perforation detected during ESD. Delayed perforation was defined as perforation diagnosed after ESD. Delayed bleeding was defined as bleeding requiring endoscopic hemostasis after ESD. En bloc resection was defined as one-piece resection that contained all neoplastic areas. R0 resection was defined as en bloc resection with negative horizontal and vertical margins.
Statistical analysis
Propensity score matching analysis was performed to reduce the effects of confounding factors. Intraoperative perforation rate was the primary outcome, while procedure time and R0 resection rate were the secondary outcomes. According to previous studies; lesion location, tumor size, and presence of preoperative biopsy were associated with long procedure times and adverse events.13-16 Therefore, the propensity score of each case was calculated using a logistic regression model based on the location of the lesion (bulb or descending/transverse part, oral side or anal side of VaterŌĆÖs papilla, and anterior/medial or lateral/posterior wall), tumor size, macroscopic type (elevated or depressed), and presence of preoperative biopsy. A one-to-one matching of S-O and control group patients was performed using the nearest neighbor method with a 0.2 caliper width of the standard deviation of the propensity score logit.
As the number of cases in each group was rather small in this study, logistic regression analysis using the propensity score as an independent variable was also performed to reveal the statistical association between the use of S-O clip and intraoperative perforation rate.
Categorical data were compared using the FisherŌĆÖs exact test or chi-squared test, and continuous data were compared using the MannŌĆōWhitney U test. Statistical significance was set at p<0.05. All statistical analyses were performed using STATA ver. 16 (StataCorp.).
Ethical statements
This study was performed in accordance with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Sendai Kousei Hospital (No. 4-49). Written informed consent for the treatment procedures was obtained from all patients.
RESULTS
Baseline characteristics of patients and lesions before and after propensity score matching
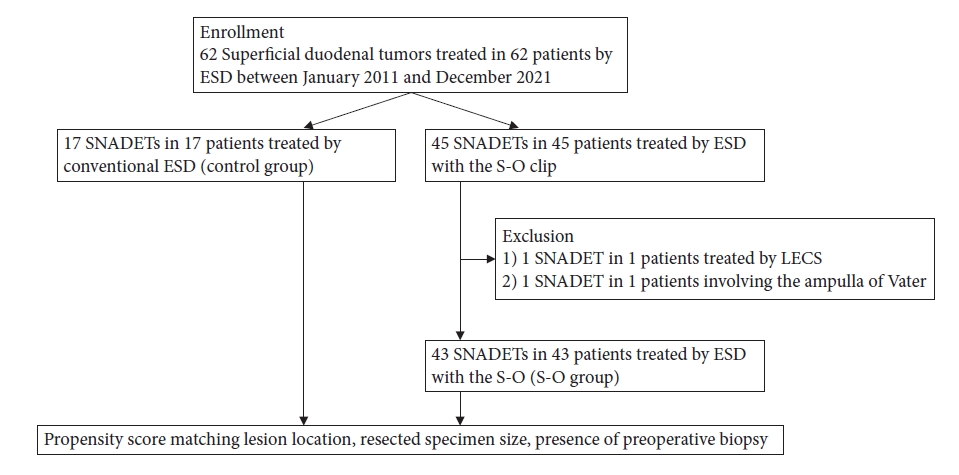
A flowchart of patient enrolment is presented in Figure 3. Sixty patients with SNADETs who were treated with ESD were finally enrolled in this study (S-O group: 43 patients vs. control group: 17 patients).
The characteristics of enrolled patients and their lesions are presented in Table 1. No significant differences were observed in the background characteristics between the groups except in pathological diagnosis and lymphovascular invasion. The control group had significantly more cases of submucosal and lymphovascular invasion than the S-O group.
After propensity score matching, 16 pairs were created between the S-O and control groups. Acceptable discrimination (C-statistic: 0.726) was observed in the propensity score model. The patient and lesion characteristics were almost similar to those before matching. No significant differences were observed in pathological diagnosis and lymphovascular invasion (Table 2).
Treatment outcomes and adverse events
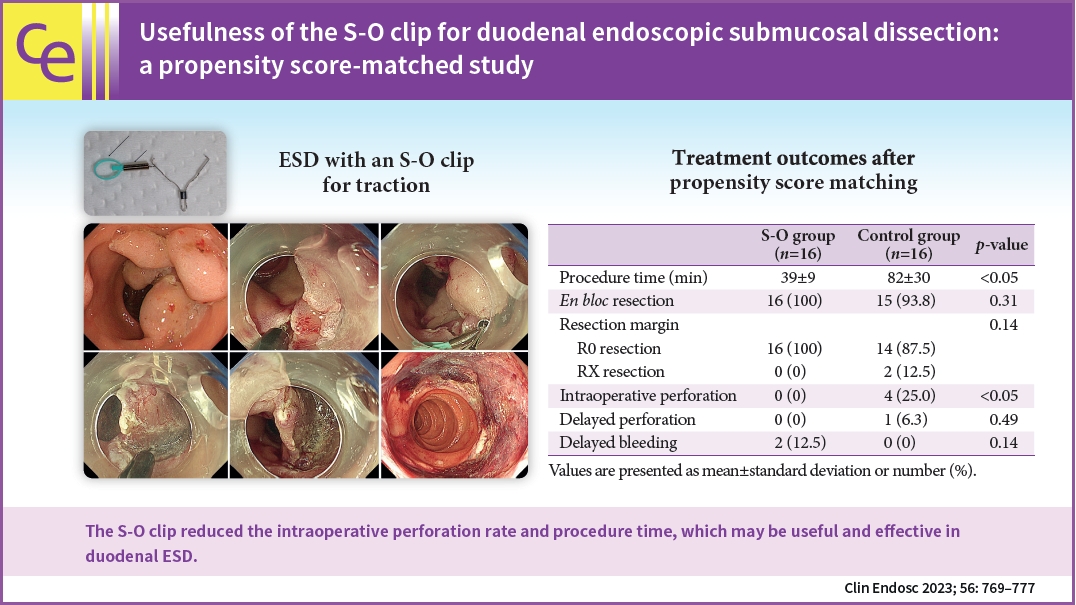
The treatment outcomes before and after matching are presented in Tables 3 and 4, respectively. Some large lesions required two S-O clips during ESD to maintain good traction. In case of large lesions, the intraoperative perforation rate in the S-O group was significantly lower than that in the control group (p=0.033) after propensity score matching. Moreover, the procedure time was significantly shorter in the S-O group than in the control group (39┬▒9 minutes vs. 82┬▒30 minutes, p=0.0037). No significant differences were noted in the rates of en bloc resection, R0 resection, delayed perforation, and delayed bleeding. These results were further confirmed before propensity score matching.
Multivariate logistic regression analysis using the propensity score
Logistic regression analysis showed that the intraoperative perforation rate was significantly lower in the S-O group than in the control group (odds ratio, 0.078; 95% confidence interval, 0.006ŌĆō0.870; p=0.03) (Table 5).
DISCUSSION
This is the first study to reveal the usefulness of the S-O clip in duodenal ESD for SNADETs using a propensity score matching analysis. The S-O clip reduced the incidence of intraoperative perforation and also reduced the procedure time by 40 minutes due to adequate traction and a clear visualization of the submucosa.
Due to the thin duodenal mucosa and poor endoscope maneuverability, the technique for duodenal ESD remains extremely difficult, often resulting in a high intraoperative perforation rate (31.3%ŌĆō75%), which is significantly higher than that associated with ESD in other parts of the gastrointestinal tract.13,17 Most of the cases of intraoperative perforation were successfully managed with conservative treatment alone. However, some cases required conversion to surgery. According to a survey conducted by the Japan Gastroenterological Endoscopy Society, the rate of surgical conversion in duodenal ESD is as high as 5.4%.18 In our study, a patient with an intraoperative perforation immediately underwent surgery, as it was impossible to close the perforation site endoscopically. Another patient in our study developed a retroperitoneal abscess due to an intraoperative perforation and was hospitalized for 67 days. Therefore, it is necessary to establish an endoscopic technique to overcome the technical challenges and risks of intraoperative perforation associated with ESD for SNADETs.
Tashima et al.19 reported the effectiveness of traction-assisted ESD using a clip-and-thread method for the treatment of duodenal lesions. Although the clip-and-thread method is technically simple and does not require special equipment, it is difficult to obtain good traction with this technique for lesions located in certain positions. Lesions of the anterior/medial wall do not have a good traction effect, since the thread is anchored along the anterior/medial wall of the duodenum. Therefore, 81% of the patients in this study had lateral/posterior lesions. In contrast, the S-O clip may be effective in all duodenal lesions, since it allows favorable determination of the direction of traction, which is a major advantage. Regardless of the position of the lesion, traction can be applied in a direction opposite to that of the lesion. This allows clear visualization of the submucosal layer and safe dissection. Therefore, the traction method using an S-O clip might be better than the clip-and-thread method in duodenal ESD.
Another method for gaining traction during duodenal ESD, the rubber band traction technique, was reported by Nabi et al.20 The basic procedure is almost similar to the S-O clip traction method and its usefulness has been demonstrated. Specific approach to gain traction should be selected based on individual endoscopistŌĆÖs preference and familiarity.
The S-O clip was initially developed for colonic ESD and reduced the procedure time.8 Similarly, the present study proved that the procedure time of duodenal ESD using the S-O clip was significantly shorter than that without the S-O clip. In general, conventional duodenal ESD without traction methods requires a complex strategy,21 and after a mucosal incision from the oral side of the lesion, creation of a mucosal flap is necessary to place the endoscope in the submucosa with slight submucosal dissection. Subsequently, circumferential incision and submucosal dissection are performed. However, the techniques required for this procedure such as creation of a mucosal flap and submucosal dissection are generally time-consuming and challenging due to the thin duodenal mucosa. However, using the S-O clip does not require a complicated treatment strategy. In the present study, a circumferential incision was made and the S-O clip was then attached to the edge of the oral side without creating a mucosal flap. Thus, submucosal dissection was performed under clear visualization of the submucosal layer. These factors may have resulted in the shorter procedure time in the S-O group.
In the present study, a hook knife was used as the device for ESD. However, Dohi et al. reported the usefulness of a scissor-type knife in duodenal ESD.21 Use of this device with the S-O clip could constitute a more stable and safer treatment technique.
The traction method using an S-O clip has several limitations. Another regular clip holding the nylon loop on the S-O clip was pulled to the proximal duodenal wall. Thus, if the lesion is in the first segment of the duodenum, the second clip is sometimes attached to the gastric wall, which was found useful in this study. The usefulness of an S-O clip anchored to the distal side has not been evaluated. However, we believe that an S-O clip anchored to the proximal side is more effective than that anchored to the distal side, since the traction effect continues throughout the dissection procedure. If the S-O clip is anchored to the distal side, the traction effect gradually weakens as submucosal dissection proceeds. Although the mean time to attach the S-O clip was 2 to 3 minutes, it took more than 5 minutes in a few cases. The duodenum is a deep and curved organ with a narrow lumen, which leads to poor scope maneuverability. Due to these factors, the endoscope can slip into the stomach when another clip that anchors the nylon loop of the S-O clip is attached to the proximal side, which does not commonly occur in colonic procedures. Moreover, if the spring of the S-O clip is stretched by >8 cm during this process, it can break. Thus, it is critical to manipulate the scope more carefully and slowly during the attachment step in duodenal procedures than in colonic procedures. Furthermore, the duodenum has a narrower lumen than the colon. Therefore, for duodenal use, it is advisable to modify the design of the S-O clip such that the spring of the clip is shortened.
Our study has several limitations. This was a retrospective study conducted at a single center with a small number of patients. However, we used propensity score matching to minimize the effects of confounding factors. Nevertheless, the results of the present study should be validated prospectively, using a larger sample size. In the current study, various methods such as clip closure, polyglycolic acid sheets, and ENPBD tubes were used to treat mucosal defects after ESD. However, the effectiveness of these methods was not evaluated in the present study. Further studies on preventive measures against adverse events after duodenal ESD are required. A greater number of lesions had invaded the submucosal layer in the control group than in the S-O group. Submucosal lesions can cause fibrosis, which may lead to longer procedure times. Although we used propensity score matching in this study, the standardized difference was higher than 0.1 for some variables. Thus, a multivariate logistic regression analysis using propensity score as an independent variable was also performed. These two analyses showed the same results: the use of an S-O clip was significantly associated with safe ESD without intraoperative perforation. The study spanned approximately 10 years and most of the S-O clip procedures were performed in the latter study period, while the conventional ESD procedures (control) were performed in the former period. Moreover, most of the ESD procedures in the control group were performed by M.N., while those in the S-O group were performed by D.H., Thus, operator differences constituted an important confounding factor in this study. However, both the operators are expert endoscopists having nearly equivalent experience in ESD (over 1,000 cases). In addition, the procedure times and adverse event rates of the two operators were not significantly different (Supplementary Table 1). Hence, we believe that operator differences did not have a significant impact on the procedure time or complication rates.
In conclusion, duodenal ESD using S-O clip traction is feasible and effective. Our findings should be confirmed in a prospective multicenter study with a large sample size.