Impact of sarcopenia on biliary drainage during neoadjuvant therapy for pancreatic cancer
Article information
Abstract
Background/Aims
Since the usefulness of neoadjuvant chemo(radiation) therapy (NAT) for pancreatic cancer has been demonstrated, recurrent biliary obstruction (RBO) in patients with pancreatic cancer with a fully covered self-expandable metal stent (FCSEMS) during NAT is expected to increase. This study investigated the impact of sarcopenia on RBO in this setting.
Methods
Patients were divided into normal and low skeletal muscle index (SMI) groups and retrospectively analyzed. Patient characteristics, overall survival, time to RBO (TRBO), stent-related adverse events, and postoperative complications were compared between the two groups. A Cox proportional hazard model was used to identify the risk factors for short TRBO.
Results
A few significant differences were observed in patient characteristics, overall survival, stent-related adverse events, and postoperative complications between 38 patients in the normal SMI group and 17 in the low SMI group. The median TRBO was not reached in the normal SMI group and was 112 days in the low SMI group (p=0.004). In multivariate analysis, low SMI was the only risk factor for short TRBO, with a hazard ratio of 5.707 (95% confidence interval, 1.148–28.381; p=0.033).
Conclusions
Sarcopenia was identified as an independent risk factor for RBO in patients with pancreatic cancer with FCSEMS during NAT.
INTRODUCTION
The usefulness of neoadjuvant chemo(radiation) therapy (NAT) in pancreatic cancer (PC), for which curative resection is planned, has been demonstrated.1-3 Preoperative biliary drainage is becoming increasingly important for PC with distal malignant biliary obstruction (MBO). Regarding the selection of stents during NAT for PC in anticipation of curative resection, previous studies reported that a self-expandable metal stent (SEMS) was superior to a plastic stent.4-9 The 2019 edition of the clinical guidelines for PC issued by the Japan Pancreas Society recommended the use of SEMS, particularly a fully covered SEMS (FCSEMS), because it is removable. However, the risk factors for recurrent biliary obstruction (RBO) in patients with PC with preoperative bile drainage by FCSEMS during NAT have not yet been examined in detail.
Duodenal invasion,10 elevated total bilirubin before SEMS insertion,11 and chemotherapies12 have been identified as risk factors for RBO after SEMS insertion. Sarcopenia has recently been reported as a risk factor for RBO after SEMS insertion in patients with unresectable MBO.13
Therefore, the present study aimed to investigate the impact of sarcopenia on RBO in patients with PC with an FCSEMS during NAT.
METHODS
Study design and patients
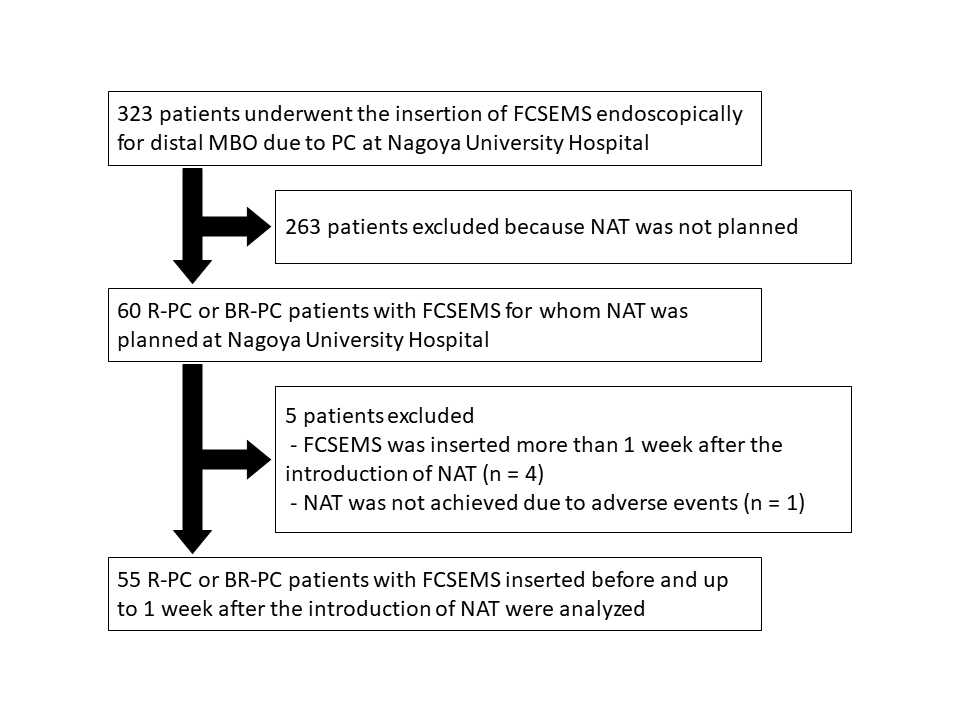
Endoscopic insertion of an FCSEMS for distal MBO due to PC was performed between May 2010 and December 2021 in 323 patients with various stages of PC who received various anticancer treatments at Nagoya University Hospital. Among these patients, 60 had resectable PC (R-PC) or borderline resectable PC (BR-PC) and NAT was planned. Resectability was reassessed in all cases according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines), ver. 1, 2020. Four patients in whom the FCSEMS was inserted more than 1 week after the introduction of NAT and one for whom NAT was not possible due to stent-related adverse events were excluded. Therefore, the remaining 55 patients with R-PC and BR-PC, and FCSEMS insertion before and up to one week after the introduction of NAT were analyzed (Fig. 1). The patients were followed up until October 2022.
Skeletal muscle index calculation method and grouping
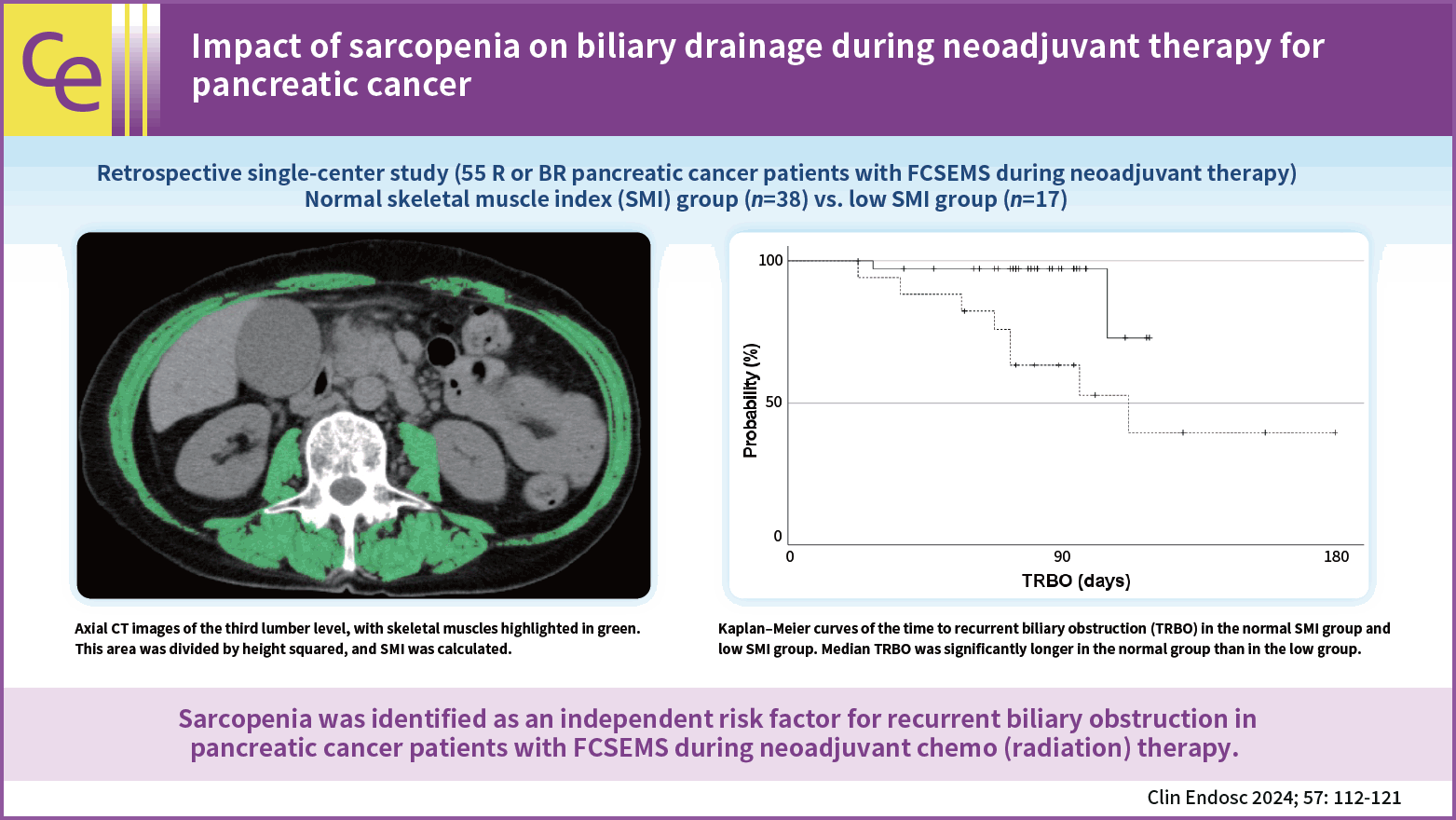
The sum of the cross-sectional areas of the skeletal muscles at the third lumbar level was measured using CT in the month before the insertion of the FCSEMS using SYNAPSE VINCENT software (Fujifilm, Tokyo, Japan). This value was then divided by the height squared, and skeletal muscle index (SMI) was calculated (Fig. 2) based on the sarcopenia assessment criteria of the Japan Society of Hepatology.14 Sex-specific SMI cut-off values were selected from a receiver operating characteristics (ROC) curve according to the highest accuracy in relation to RBO. Patients were assigned to one of the following two groups using the cut-off values: normal SMI and low SMI.
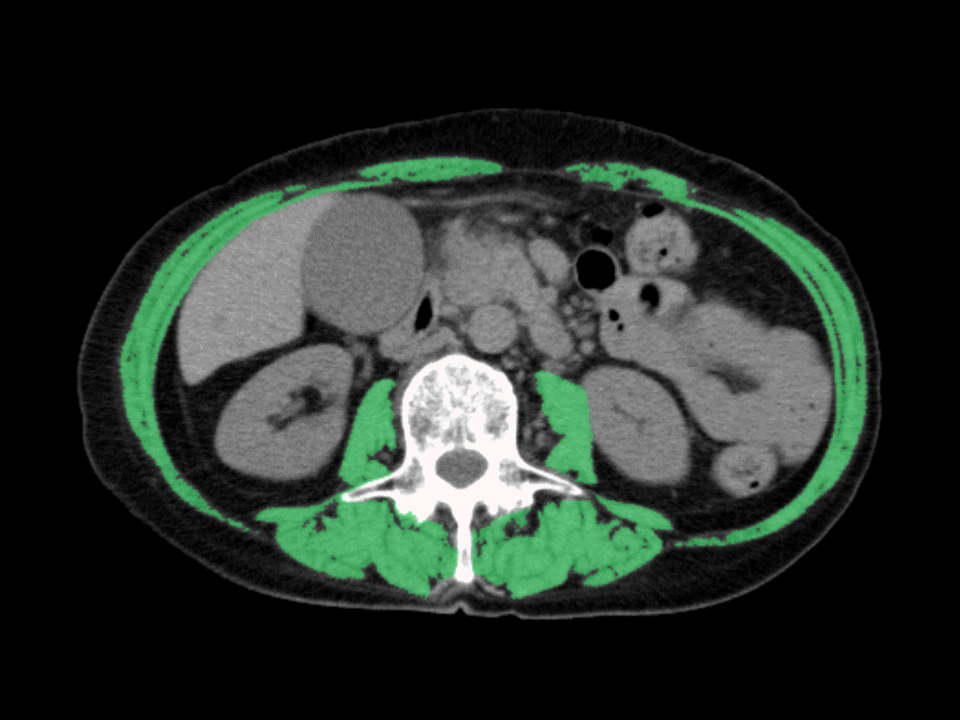
Axial computed tomography images at the third lumbar level, with skeletal muscles highlighted in green (–29 to 150 Hounsfield units) using SYNAPSE VINCENT software (Fujifilm).
Patient characteristics, overall survival (OS), time to RBO (TRBO), causes and management of RBO, stent-related adverse events, and complications after curative resection were retrospectively compared between the normal and low SMI groups. Postoperative complications were evaluated using the Clavien-Dindo classification,15 with Grade IIIa or higher being severe.
FCSEMS insertion
FCSEMS was inserted endoscopically in a normal manner. The placement involved extension above the top of the stricture by at least 10 mm and into the duodenum by approximately 10 mm. The following FCSEMS were inserted: WallFlex Biliary RX Fully Covered Stents in 52 patients (Boston Scientific) and BONASTENTs in three patients (Standard Sci-Tech Inc.). FCSEMS with diameters of 8 and 10 mm were used. The type and diameter of the SEMS were selected at the discretion of each endoscopist. Antibiotics were administered to all patients.
Definition of stent-related adverse events
The Tokyo Criteria 2014 was employed to define and grade stent-related adverse events.16 Stent dysfunction was suspected in patients with elevated liver enzyme levels above baseline values and those with biliary dilation on imaging modalities or endoscopic findings indicating occlusion or migration of the stent. As difficulties were sometimes associated with distinguishing between food impaction and sludge, these two factors were combined in the present analysis. Patients in whom reintervention revealed a completely or partially migrated FCSEMS were diagnosed with stent migration. RBO was defined as the composite endpoint of occlusion or migration. In patients with RBO attributed to both occlusion and migration, migration was considered the cause. The TRBO of the FCSEMS was calculated as the time between stent placement and dysfunction.
Response evaluation in primary tumors
Since only primary tumors are considered to affect RBO, only the increase or shrinkage of the primary tumor diameter at the time of RBO, surgical resection, or the non-resection decision was evaluated according to the revised response evaluation criteria in the solid tumor guidelines (ver. 1.1), with the primary tumor diameter at the time of FCSEMS insertion as a reference. The definitions are as follows. Complete response (CR): disappearance of the primary tumor. Partial response (PR): at least a 30% decrease in the diameter of the primary tumor. Progressive disease (PD): at least a 20% increase in the diameter of the primary tumor. Stable disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Statistical analysis
For comparisons between two groups, categorical variables were analyzed using Fisher exact test or the chi-squared test, and continuous variables were analyzed using the Mann-Whitney U-test. Cumulative OS and TRBO were estimated using the Kaplan-Meier method and supplemented by the log-rank test for comparisons between groups. At the time of analysis, patients without RBO were censored as of the date of surgical resection or the non-resection decision. A Cox proportional hazards model was used to identify risk factors for RBO. Each continuous variable was categorized into two groups based on the median value, except for SMI. Risk factors with a p-value <0.20 in the univariate analysis were examined in a multivariate analysis, and the hazard ratio (HR) and 95% confidence interval (CI) were calculated. The significance of differences was set at a p-value <0.05. Statistical analyses were performed using IBM SPSS Statistics ver. 28.0 (IBM Corp.).
Ethical statements
The Ethics Committee of Nagoya University Hospital approved this single-center retrospective analysis (approval number: 2016-0032), which was conducted in accordance with the guidelines of the Declaration of Helsinki for biomedical research involving human subjects (clinical trial registration number: UMIN000025631). The research content was described, and contact information was provided in an opt-out format on our hospital website for patients who did not wish to participate.
RESULTS
Patient characteristics
Table 1 shows a summary of the patient characteristics. The median age of the patients was 67 years, and there were 28 males and 27 females. The Eastern Cooperative Oncology Group performance status (PS) was good, with 52 and three patients having PS 0 and 1, respectively. There were 27 and 28 patients with R-PC and BR-PC, respectively. At the time of FCSEMS insertion, 48 patients had plastic stents, and 18 had cholestasis. Cholestasis referred to patients in whom the plastic stent placement caused RBO at the time of FCSEMS insertion and those in whom FCSEMS insertion was performed as the first biliary drainage. FCSEMS with a diameter of 8 mm and length of 6 cm were used. NAT regimens varied, with 11 patients receiving radiation therapy (RT). Response evaluation of the primary tumors was PD, SD, PR, and CR in 6, 40, 9, and 0 patients, respectively. Prehabilitation (preoperative physical and nutritional support) was performed for 21 patients. Forty-two patients underwent curative resections. Of these, 30 patients underwent R0 resection, and the Evans grade (histopathological therapeutic effects of NAT) was I in six patients, IIa in 26, IIb in eight, III in one, and IV in one. The median time to the date of surgical resection or non-resection decision was 86 days (range, 38–180 days).
The median SMI were 41.9 (range, 32.5–70.2) and 35.1 (22.4–47.6) cm2/m2 in males and females, respectively. SMI cut-off values were 38.0 (area under the ROC curve, 0.680) and 35.0 (area under the ROC curve, 0.650) cm2/m2 in males and females, respectively. Patients were classified into the normal SMI group (n=38) and the low SMI group (n=17) using these cut-off values. Significant differences were observed in sex and the time to the date of surgical resection or the non-resection decision between the normal SMI and low SMI groups, with significantly fewer males (24/38 [63.2%] vs. 4/17 [23.5%], p=0.009) and a significantly longer time to the date of surgical resection or the non-resection decision (80 [38–119] vs. 98 [58–180], p=0.013) observed in the low SMI group.
OS and TRBO between normal SMI and low SMI groups
The median follow-up period was 516 days and the median OS was 689 days. Figure 3 shows the Kaplan-Meier curves for OS in the normal and low SMI groups. The median OS was 689 and 1,059 days in the normal and low SMI groups, respectively, with no significant difference between the two groups (p=0.701).
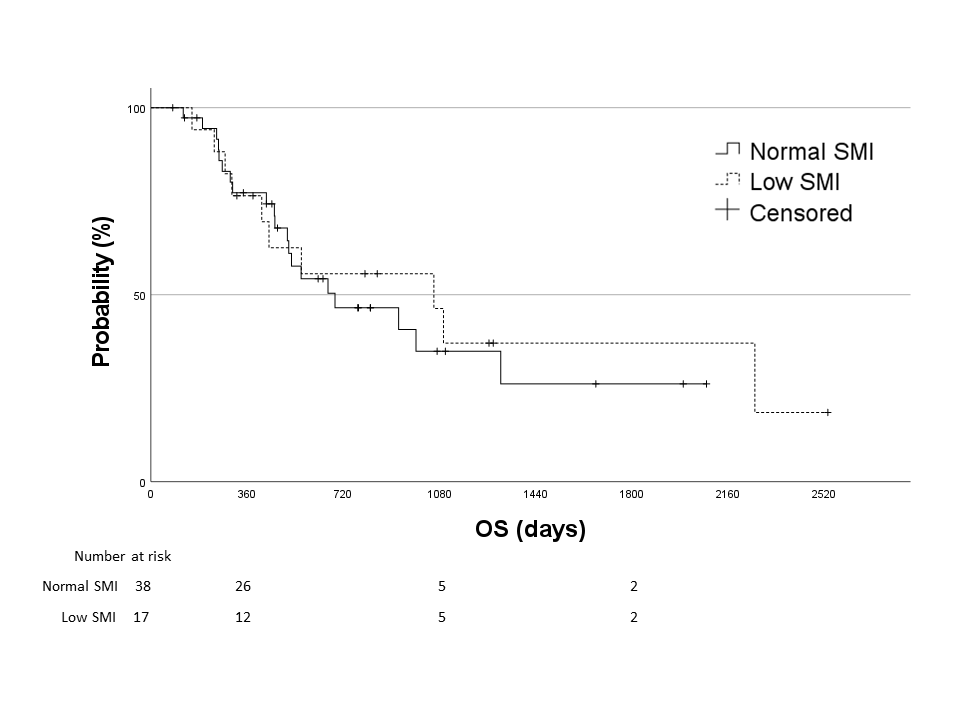
Kaplan-Meier curves of the overall survival (OS) in the normal skeletal muscle index (SMI) group (solid line) and low SMI group (dotted line). Median OS was 689 days in the normal SMI group and 1,059 days in the low SMI group, with no significant difference between the two groups (the log-rank test, p=0.701).
Figure 4 shows Kaplan-Meier curves for TRBO in the two groups. Median TRBO was not reached in the normal SMI group and was 112 days in the low SMI group, and, thus, was significantly longer in the normal SMI group than in the low SMI group (p=0.004).

Kaplan-Meier curves of the time to recurrent biliary obstruction (TRBO) in the normal skeletal muscle index (SMI) group (solid line) and low SMI group (dotted line). Median TRBO was not reached in the normal SMI group and was 112 days in the low SMI patients, which was significantly longer in the normal SMI group than in the low SMI group (the log-rank test, p=0.004).
Risk factors for short TRBO
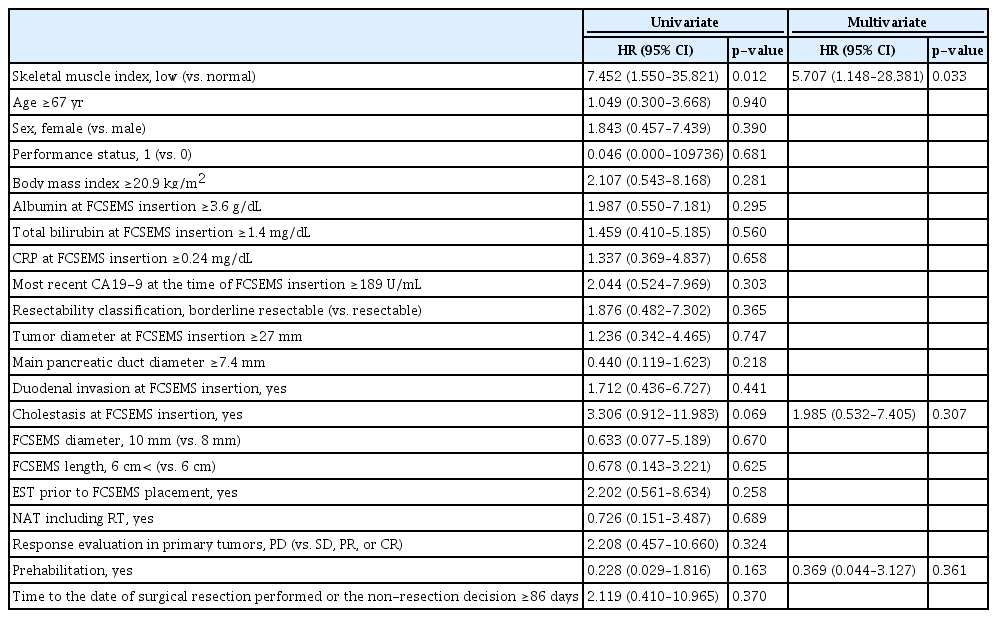
The univariate analysis of potential risk factors for short TRBO identified three factors with a p-value <0.20: SMI, cholestasis at FCSEMS insertion, and prehabilitation. In a multivariate analysis of these three factors, only SMI remained significant (p=0.033). In comparison with normal SMI, the risk of RBO was significantly higher with a low SMI, with an HR of 5.707 (95% CI, 1.148–28.381) (Table 2).
RBO and stent-related adverse events
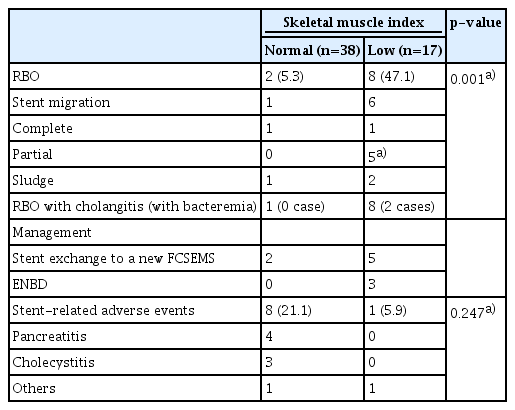
Table 3 lists the causes of RBO. The RBO rate was significantly higher in the low SMI group (47.1%, 8/17) than in the normal SMI group (5.3%, 2/38; p=0.001). The main cause of RBO was stent migration, which was accompanied by sludge in all five partial migration cases. For the two cases of complete migration, the involvement of sludge was unknown. RBO was managed using stent exchange to a new FCSEMS or endoscopic nasobiliary drainage for the bridge to surgery. The latter was selected when the waiting time before the surgery was short.
Table 3 shows stent-related adverse events. The rates of stent-related adverse events in the normal SMI and low SMI groups were 21.1% (8/38) and 5.9% (1/17), respectively, and did not significantly differ (p=0.247). Pancreatitis and cholecystitis developed in four and three patients, respectively, in the normal SMI group, and in no patients in the low SMI group. All four cases of pancreatitis were mild and rapidly improved with conservative treatment. Contrarily, one of the three cholecystitis cases was mild, while two were moderately severe and required percutaneous transhepatic gallbladder drainage.
Postoperative complications
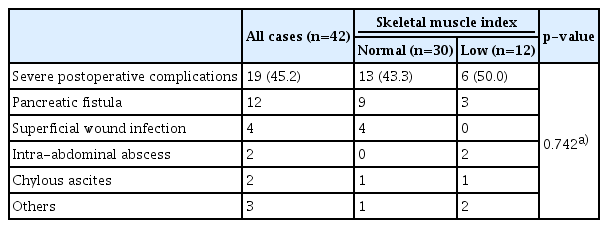
Details of postoperative complications are shown in Table 4. Severe postoperative complications were observed in 19 of 42 patients who underwent curative resection. No significant differences were observed between the normal and low SMI groups (13/30 [43.3%] vs. 6/12 [50.0%], p=0.742). Postoperative mortality was not observed.
DISCUSSION
The usefulness of NAT for PC for which curative resection is planned has been demonstrated,1-3 and RBO is expected to increase in patients with PC drained by FCSEMS during NAT. Although the risk factors for RBO in this setting remain unclear, to the best of our knowledge, the present study is the first to identify a low SMI as an independent risk factor.
Regarding stent selection, previous studies suggested that SEMS were superior to plastic stents.4-9 It is important to note that SEMS included not only FCSEMS but also partially covered and uncovered SEMS. An international prospective multicenter trial on 119 patients with PC reported similar findings for FCSEMS and uncovered SEMS. In that study, significant predictors of a failure to decompress biliary obstruction were a SEMS length of 4 cm, but not 6 or 8 cm, and the presence of the gallbladder.17 In a prospective multicenter study of 26 patients with PC that evaluated the safety and efficacy of partially covered SEMS, NAT including RT was suggested to increase the risk of RBO.18 In the present study, FCSEMS with a length of 4 cm was not used, and only three patients had undergone cholecystectomy. Furthermore, NAT, including RT, was not a significant risk factor.
The European Society of Gastrointestinal Endoscopy recommends SEMS with a diameter of 10 mm.19 The reason for this was not explicitly stated but may be due to the majority of studies using SEMS with a diameter of 10 mm for PC during NAT. In contrast, an FCSEMS with a diameter of 8 mm was placed in 50 of the 55 patients in the present study. Although an FCSEMS with a diameter of 10 mm was used in a small number of cases, RBO rates were 18.0% (9/50) and 20.0% (1/5) for 8 mm and 10 mm, respectively, with no significant difference (p>0.999). The RBO rates for SEMS with a diameter of 10 mm (including FCSEMS and partially covered SEMS) in previous studies were 11.1% (1/9),4 23.5% (4/17),5 25.0% (4/16),6 17.6% (3/17),7 27.8% (15/54) (including two cases of FCSEMS with a diameter of 8 mm),17 5.9% (1/17),8 34.6% (9/26),18 and 14.8% (4/27).9 Although comparisons are difficult due to differences in the methods used to evaluate RBO rates, follow-up periods, and patient characteristics, the present results on FCSEMS with a diameter of 8 mm appear to be consistent with those of SEMS with a diameter of 10 mm. Consistent with the results of our randomized controlled trial comparing FCSEMS with diameters of 8 and 10 mm in patients with unresectable MBO,20 similar findings may be obtained for FCSEMS with diameters of 8 and 10 mm for PC during NAT.
Several diseases, including malignancies and chronic liver disease, have been implicated in the development of sarcopenia, which is characterized by the degenerative loss of skeletal muscle mass and strength.14,21,22 The psoas muscle mass index (PMI) is an objective and quantitative marker for assessing skeletal muscle mass.23 Previous studies that reported a relationship between sarcopenia and a poor prognosis after resection and increased susceptibility to RBO in pancreatobiliary cancer used PMI as a measure of sarcopenia.13,24,25 In the present study, SMI was used for greater precision according to the sarcopenia assessment criteria of the Japan Society of Hepatology.14 Cut-off values for SMI were derived from ROC curve analyses, which is the most accurate measurement associated with RBO, and were set as 38.0 and 35.0 cm2/m2 in males and females, respectively. In contrast, the cut-off values of the Japan Society of Hepatology are 42 and 38 cm2/m2 in males and females, respectively.14 The use of these cut-off values in the present study resulted in 34 patients being assigned to the low SMI group, which represented a two-fold increase. PC with distal biliary obstruction appears to cause muscle atrophy, even in relatively early-stage lesions such as R-PC and BR-PC. One potential contributing factor is the presence of pancreatic exocrine insufficiency associated with main pancreatic duct obstruction.26-28 Although pancreatic exocrine function was not investigated in the present study, main pancreatic duct dilation, reflecting tumor-induced obstruction, was observed in most cases.
Sarcopenia has recently been reported as a risk factor for RBO after SEMS placement for patients with unresectable MBO.13 It has been hypothesized that patients with advanced sarcopenia are less physically active; less activity is more likely to be accompanied by impaired gallbladder motility, which, in turn, increases the prevalence of gallstones and sludge,29,30 and, thus, the risk of RBO.13 In the present study, RBO was caused by sludge and stent migration. Since all partial stent migration cases accompanied stent occlusion by sludge, sludge was involved in most cases. However, patients in the present study generally had a good PS; therefore, this hypothesis may not be the only explanation. Contrarily, sarcopenia has been suggested to play a role in impaired immunity,31 which may increase the susceptibility of patients with PC drained by FCSEMS to cholangitis. Furthermore, anticancer treatment, including NAT, may promote the formation of sludge through the generation of biofilms in the FCSEMS lumen, which, when combined with impaired immunity caused by NAT, increases the risk of developing cholangitis.12 In the present study, all eight patients with RBO in the low SMI group developed infections, and two had bacteremia, while only one out of two patients with RBO in the normal SMI group had cholangitis and none developed bacteremia (Table 3).
Prehabilitation has attracted increasing attention with the main goal of improving postoperative outcomes; however, limited information is currently available for patients with PC, including those undergoing NAT, and issues remain regarding the selection of appropriate cases with a limited number of physical therapists.32 At our institution, we reported the efficacy of prehabilitation in patients undergoing hepato-pancreato-biliary upfront surgeries for malignancy. This study demonstrated that the introduction of prehabilitation was associated with better physical fitness, preserved nutritional status, and shorter postoperative hospital stay, with a median waiting period of only 32 days. In addition, a longer duration of prehabilitation was associated with better effects.33 Patients with PC undergoing NAT may benefit more from prehabilitation because of the long waiting period. Prehabilitation may also effectively prevent RBO by reducing gallbladder sludge formation by exercise and improving immunocompetence by attenuating sarcopenia. Although the present study did not show the preventive effects of prehabilitation on RBO, it is important to note that only 21 patients underwent prehabilitation and only five had a low SMI.
There are several limitations that need to be addressed because of the retrospective nature of this analysis and the small sample size. The FCSEMS type and diameter (8 mm or 10 mm) were not predefined during the study period. However, heterogeneity in the FCSEMS type and diameter is unlikely to have influenced the main results because 8-mm WallFlex Biliary RX Fully Covered Stents were the most preferentially placed. Furthermore, NAT regimens are heterogeneous, and the impact of various NAT regimens on RBO remains unknown because of the limited number of patients. Another limitation was that SMI alone was evaluated as an indicator of sarcopenia. Although the degenerative loss of muscle mass is an important characteristic of sarcopenia, muscle strength (i.e., grip strength) also needs to be evaluated14, 31; however, this was not examined because this was a retrospective analysis. Furthermore, the SMI cut-off values used in this study were calculated from a small number of patients and were not generic. Therefore, a prospective multicenter study examining the effects of sarcopenia on RBO is required to validate the present results.
In conclusion, this retrospective analysis identified low SMI as an independent risk factor for RBO in patients with PC drained by FCSEMS during NAT. These patients require careful monitoring because of the high risk of RBO.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: KK, HK, EO, TI; Data curation: KK, HK, EO, TI; Formal analysis: KK, HK, EO, TI; Investigation: KK, HK, EO, TI; Methodology: KK, HK, EO, TI; Project administration: KK, HK, EO, TI; Supervision: HK; Validation: KK, HK, EO, TI, KY, YM, TI, HT, OM, JY, YY, TE, YK; Visualization: KK, HK, EO, TI; Writing–original draft: KK; Writing–review & editing: all authors.