Impact of a simple non-invasive nasal mask device on intraprocedural hypoxemia in overweight individuals undergoing upper gastrointestinal endoscopy with sedation provided by a non-anesthesiologist provider
Article information
Abstract
Background/Aims
Hypoxemia is a common side effect of propofol sedation during endoscopy. Applying mild positive airway pressure (PAP) using a nasal mask may offer a simple way to reduce such events and optimize the conditions for diagnostic and therapeutic upper gastrointestinal endoscopies.
Methods
We compared overweight patients (body mass index >25 kg/m2) with a nasal PAP mask or standard nasal cannula undergoing upper gastrointestinal endoscopies by non-anesthesiologists who provided propofol sedation. Outcome parameters included the frequency and severity of hypoxemic episodes.
Results
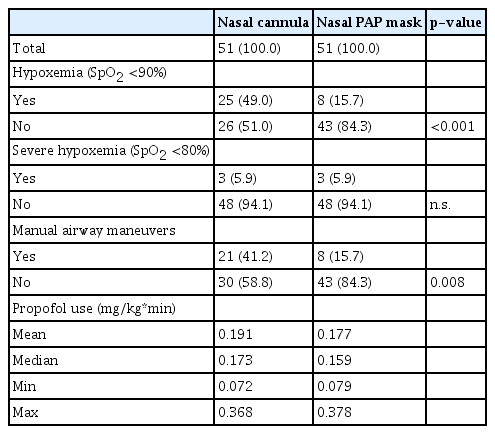
We analyzed 102 procedures in 51 patients with nasal PAP masks and 51 controls. Episodes of hypoxemia (oxygen saturation [SpO2] <90% at any time during sedation) occurred in 25 (49.0%) controls compared to 8 (15.7%) patients with nasal PAP masks (p<0.001). Severe hypoxemia (SpO2 <80%) occurred in three individuals (5.9%) in both groups. The mean delta between baseline SpO2 and the lowest SpO2 recorded was significantly decreased among patients with nasal PAP mask compared to controls (3.7 and 8.2 percentage points difference, respectively). There were significantly fewer airway interventions performed in the nasal PAP mask group (15.7% vs. 41.2%, p=0.008).
Conclusions
Using a nasal PAP mask may be a simple means of increasing patient safety and ease of examination.
INTRODUCTION
Gastrointestinal (GI) endoscopy is mostly performed under sedation to increase patient comfort and facilitate diagnostic and therapeutic procedures. A German societal guideline on sedation for GI endoscopy recommends that sedation should be or sedation would be undergoing GI endoscopy.1 The most commonly used agents are propofol, administered as an intravenous (IV) bolus and monotherapy (that is, without other agents). In healthy patients, the German and European guidelines consider the administration of propofol sedation by a gastroenterologist or nurse with specialized training to be fully adequate and safe.1,2 Anesthesiologist support is only recommended for patients with severe comorbidities, such as those categorized as American Society of Anesthesiologists (ASA) class III or higher, those with unusual anatomy predictive of a difficult airway, and those with complex endoscopic interventions.
The advantages of propofol as a sedative for GI endoscopy include short recovery and discharge times and high patient satisfaction.3 However, the side effects of propofol sedation include hypotension, arrhythmias, and hypoxemia.1 Hypoxemia during propofol sedation is likely caused by decreased respiratory drive and reduced muscle tone in the upper airway.4,5 Hypoxemia is relatively common, occurring in approximately 8.8% to 12.8% of healthy individuals undergoing GI endoscopy under propofol sedation.2,6 The risk of episodes of hypoxemia under sedation is significantly higher in individuals with a higher body mass index (BMI).7
In this study, we report using a simple nasal positive airway pressure (PAP) mask that can be connected to a standard oxygen outlet during upper GI endoscopy in overweight adults. The primary outcome parameter was hypoxemia at any time during the procedure. The results were compared to those of a historical control group that underwent comparable procedures when the nasal PAP mask was unavailable and oxygen was supplemented using a standard nasal cannula.
METHODS
Study design
We retrospectively analyzed consecutive overweight patients who underwent upper GI endoscopy at our center in August 2021, when the nasal PAP mask became available. Asklepios Hospital Barmbek is a large urban acute care and referral hospital where both inpatients and outpatients undergo endoscopic examinations.
The patients had to be ASA class I or II, 18 years of age or older, and have a BMI of 25 kg/m2 or more to be included in the analysis. Additionally, only examinations of patients in the left-lateral position were included. As per the hospital guidelines, standard sedation was performed with an IV bolus of propofol at an initial dose of 0.5 mg/kg and a subsequent dose of 20 mg as required. Continuous monitoring of the heart rate, peripheral oxygen saturation (SpO2), and intermittent blood pressure measurements were performed during the procedure. All procedures, including sedation, were performed by gastroenterologists and endoscopy nursing staff trained in sedation for GI endoscopy. Procedures in which an anesthesiologist performed sedation were not included. Individuals fulfilling the same criteria who were examined at a time when the nasal PAP mask was unavailable served as historical controls. In these patients, oxygen was supplemented using a standard low-flow nasal cannula.
Device
The nasal PAP mask used in this study was the SuperNO2VA Nasal Positive Airway Pressure Ventilation System (Vyaire). An elastic strap is used to secure this mask over the patient’s nose and behind their head (Fig. 1). The nasal PAP mask is connected to a standard oxygen outlet, and the oxygen flow is set to 8 to 10 L/min. Thus, no specific equipment besides the nasal PAP mask and oxygen outlet is required. The controls received oxygen through a standard single-nostril nasal cannula at a flow rate of 2 L/min. The mouthpieces used during the procedure were identical in the nasal and control groups. The type and duration of the procedure; the amount of propofol used; oxygen saturation; adverse events such as hypoxemia (SpO2 <90%); and the need for manual airway maneuvers (chin lift, jaw thrust), bag or mask ventilation, or endotracheal intubation were all evaluated.

Nasal positive airway pressure mask used in this study during upper gastrointestinal endoscopy. SuperNO2VA Nasal Positive Airway Pressure Ventilation System (Vyaire). (A) Positioning and attachment of the device. (B) Upper gastrointestinal endoscopy with the nasal positive airway pressure mask in place.
Statistics
Data were collected in Microsoft Excel and were analyzed using IBM SPSS Statistics ver. 25.0 software (IBM Corp.). Depending on the particular data set, the Student t-test, Mann-Whitney test, Wilcoxon rank sum test, and Pearson’s chi-square test were used to test for statistical significance. Continuous data are summarized as means±standard deviations and medians (25th and 75th percentiles). Categorical data are presented as number (%). Differences in outcome data between the nasal PAP mask and standard mask were analyzed with the Wilcoxon-Mann Whitney test for continuous data and Pearson’s chi-square test for categorical data. Differences in SpO2 values from baseline were compared using the Wilcoxon signed-rank test. All p-values were two-sided, and a p-value <0.05 was considered significant. All calculations were performed using the statistical analysis software R ver. 3.5.1 (R Foundation for Statistical Computing).
Ethical statements
This analysis was registered with the Hamburg (Germany) Chamber of Physicians ethics board. A formal vote or patients Written informed consent was waivered because of the purely retrospective anonymized study design (reference number 2022-300236-WF).
RESULTS
A total of 51 upper GI endoscopies in which patients received oxygen supplementation via a nasal PAP mask were compared with those in which oxygen was supplemented via a standard nasal cannula. The two groups were comparable in terms of age, sex, BMI, and types of upper GI endoscopies performed (Table 1). A significantly higher number of combined procedures (e.g., esophagogastroduodenoscopy [EGD] plus endoscopic ultrasound) were performed in the nasal PAP mask group (17.6% vs. 2.0%). There was a higher number of individuals with known respiratory disease in the nasal PAP mask group than in the control group (19.6% vs. 5.9%). However, the difference was not statistically significant, and only two individuals in the nasal PAP group required oxygen before sedation.
All patients had a baseline SpO2 >90% prior to sedation, and the baseline SpO2 was not significantly different between the two groups. An episode of hypoxemia (SpO2 <90% at any point during sedation) occurred in 25 individuals (49.0%) in the control group compared with 8 (15.7%) in the nasal PAP mask group (Table 2). This difference was highly significant (p<0.001). The difference between SpO2 before sedation and the lowest recorded SpO2 during sedation was significantly greater in the control group than in the nasal PAP mask group (Fig. 2). Severe hypoxemia, that is, a SpO2 <80% at any point during sedation, occurred in three individuals (5.9%) in the nasal PAP mask group and three individuals (5.9%) in the control group. All episodes of hypoxemia could be reversed by a combination of manual airway maneuvers such as chin lift and jaw thrust. In the control group, oxygen flow over the nasal cannula was usually increased in parallel. In line with the higher rate of hypoxemia events, airway maneuvers were used significantly more frequently in the control group than in the nasal PAP mask group. There were no events in the series in which bag and mask ventilation, endotracheal intubation, or cardiopulmonary resuscitation were performed. There were no periprocedural deaths or admissions to the intensive care unit.
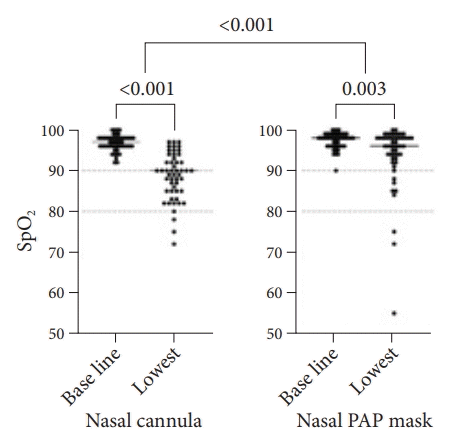
Oxygen saturation before and during sedation. Oxygen saturation (SpO2) before sedation (baseline) and lowest value during sedation (lowest) in patients with nasal cannula compared to those in the nasal positive airway pressure (PAP) mask cohort. Within each group, differences were tested for statistical significance using Wilcoxon signed ranks test; the difference in delta-SpO2 between the groups was tested using two-sided Wilcoxon-Mann-Whitney test.
The amount of propofol used, when normalized to patient weight and procedure duration, was numerically higher in the control group than in the nasal PAP mask group; however, this difference was not statistically significant.
DISCUSSION
In this retrospective series, we found that in a cohort of overweight patients undergoing upper GI endoscopy with a nasal PAP mask as a means to supplement oxygen, there were fewer episodes of hypoxemia than in a historical control cohort in which oxygen was supplemented using a standard nasal cannula. Additionally, the decrease in SpO2 observed during sedation was reduced.
The two groups were largely comparable in terms of most baseline characteristics; however, there were more individuals with pulmonary comorbidities in the nasal PAP mask group. Numerically, the patients in this group had a higher mean BMI, and there were more inpatients in this group, but these differences were not statistically significant. Thus, individuals in the nasal PAP mask group were expected to be more prone to hypoxemia. However, there were fewer hypoxemia events in this group, and the observed decrease in SpO2 during sedation was reduced. These differences were highly statistically significant, despite the limited group size. Mechanistically, it is plausible that the higher oxygen flows in combination with an, albeit unquantified, PAP generated by the nasal PAP device helps to maintain upper airway patency and more stable oxygenation during propofol sedation. However, since this was not a prospective or randomized study, we cannot conclude that there is a causal relationship between using a nasal PAP mask and the lower number of hypoxemia episodes observed during an upper GI endoscopy. Additionally, given the study’s retrospective nature, there is a risk of selection bias in the control group. A prospective, ideally randomized study will be required to rule out this concern.
Our findings are in line with those from a smaller observational cohort of obese individuals undergoing EGD prior to bariatric surgery, where the use of the same nasal PAP mask was associated with a comparable reduction in hypoxemia events.8 In contrast to our study, sedation was provided by the anesthesiology team. In a randomized controlled study by Bai et al. of obese individuals undergoing colonoscopy, the same nasal PAP mask device was compared to nasal cannula, with hypoxemia occurring at a lower frequency (5% vs. 22%) in the nasal PAP mask group.9 Again, this is in keeping with our observations. However, there are certain crucial differences compared to the present study; in the study by Bai et al.,9 individuals underwent lower and not upper GI endoscopy, an anesthesiology team provided sedation, and higher oxygen flow rates were provided in both groups.
Manual maneuvers to maintain upper airway patency were used less frequently in the nasal PAP mask group. It is the impression of the authors that examination conditions are better in overweight patients wearing a nasal PAP mask since the team has to devote less time and focus on sedation and airway management. Episodes of severe hypoxemia (SpO2 <80%) were rare in this study but occurred with equal frequency in both groups. This may suggest that the nasal PAP mask helps prevent minor respiratory impairments due to decreased upper airway tone but is less helpful in preventing more serious respiratory impairments, such as those caused by prolonged apnea or aspiration. However, given the retrospective design of this study, these considerations remain speculative at this point.
Hypoxemia is common during endoscopy under propofol sedation, but the frequency reported in studies varies widely. In our historical control group, 49.0% of the patients had at least one episode of hypoxemia. A meta-analysis published in 2008 found that in trials with an arm where propofol alone was used for sedation during GI endoscopy, hypoxemia occurred in 11% (confidence interval, 7%–16%).10 However, the included patients tended to be healthier and most data were from colonoscopy, where the risk of hypoxemia may be lower compared to upper GI endoscopy. Moreover, the definition of hypoxemia differs among studies. In more high-risk patient groups, higher rates of hypoxemia have been reported: in a cohort of obese individuals undergoing EGD where hypoxemia was defined as SpO2 <90% for at least 15 seconds, at least one hypoxemia event occurred in 46.7% of the individuals.8 In most frail patients undergoing gastrostomy tube placement, hypoxemia rates of over 50% have been reported.11 Notably, most patients quickly recover from transient hypoxemia during endoscopy. Typically, they do not recall any discomfort, and it is unclear whether transient episodes of mild hypoxemia are clinically relevant. Nonetheless, severe or prolonged hypoxemia is harmful; thus, efforts should be made to avert hypoxemia during sedation.
Several approaches to reduce hypoxemia events during GI endoscopy have been proposed: capnography during sedation is non-invasive and allows the detection of apnea and initiation of countermeasures before hypoxemia develops.11,12 However, it has also been reported that it reduces apnea episodes but not actual hypoxemia.13 Thus, capnography might divert time and team focus to deal with minor episodes with unclear clinical relevance. Moreover, upper GI endoscopy may disturb expiratory CO2 measurements, impairing the reliability of monitoring. High-flow nasal cannula oxygen has been shown to reduce hypoxemic episodes during sedation for GI endoscopy in several randomized trials and a recent meta-analysis.14 However, this approach has been criticized because it might obscure respiratory depression and create a false sense of security.15 Finally, supraglottic airway devices such as nasopharyngeal tubes, laryngeal tubes, and laryngeal masks are being evaluated during GI endoscopy, often with modifications to the original device to accommodate endoscopy during upper GI endoscopy.16 However, these devices are invasive and require specialized training.
The nasal PAP mask used in this series is non-invasive and easy to use by non-anesthesiologists without requiring additional equipment beyond a standard oxygen outlet. Our data suggest that this may be a simple and effective way to reduce hypoxemic episodes in overweight patients undergoing upper GI endoscopy. To formally test this hypothesis, a randomized prospective trial is desirable.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: JD, JH, FB, RE, SW, TvH; Data curation: JD, JH, PW, TvH; Formal analysis: JD, JH, PW, TvH; Funding acquisition: TvH; Investigation: JH, DS, MS, HK, TB, TvH; Methodology: JD, JH, PW, FB, RE, SW, TvH; Project administration: JD, TvH; Supervision: TvH; Validation: JD, TvH; Visualization: JD, JH, TvH; Writing–original draft: JD, JH, TvH; Writing–review & editing: all authors.