Preclinical study of a novel ingestible bleeding sensor for upper gastrointestinal bleeding
Article information
Abstract
Background/Aims
Upper gastrointestinal bleeding (UGIB) is a life-threatening condition that necessitates early identification and intervention and is associated with substantial morbidity, mortality, and socioeconomic burden. However, several diagnostic challenges remain regarding risk stratification and the optimal timing of endoscopy. The PillSense System is a noninvasive device developed to detect blood in patients with UGIB in real time. This study aimed to assess the safety and performance characteristics of PillSense using a simulated bleeding model.
Methods
A preclinical study was performed using an in vivo porcine model (14 animals). Fourteen PillSense capsules were endoscopically placed in the stomach and blood was injected into the stomach to simulate bleeding. The safety and sensitivity of blood detection and pill excretion were also investigated.
Results
All the sensors successfully detected the presence or absence of blood. The minimum threshold was 9% blood concentration, with additional detection of increasing concentrations of up to 22.5% blood. All the sensors passed naturally through the gastrointestinal tract.
Conclusions
This study demonstrated the ability of the PillSense System sensor to detect UGIB across a wide range of blood concentrations. This ingestible device detects UGIB in real time and has the potential to be an effective tool to supplement the current standard of care. These favorable results will be further investigated in future clinical studies.
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) is a common, potentially life-threatening emergency that is associated with substantial morbidity and mortality and necessitates early identification and intervention. With an annualized incidence of approximately 80 to 150 per 100,000 individuals, UGIB causes a significant socioeconomic burden, representing an annual healthcare expenditure of nearly 2.5 billion United States dollar (USD) in the US.1-5 Defined as a hemorrhage originating proximal to the ligament of Treitz, UGIB’s most common etiologies consist of peptic ulcer disease, esophageal varices, and erosive conditions, such as gastritis, esophagitis, and duodenitis.6,7 Despite advances in diagnostic and therapeutic techniques, the overall mortality from UGIB has remained between 1% and 20%, averaging approximately 10%.8-17 Several studies have reported mortality rates of approximately 30%, especially in inpatient hospital settings.9,18-20 Furthermore, UGIB increases the risk of developing several potentially life-threatening complications such as hypovolemic shock, acute respiratory failure, and acute renal failure, especially in patients with additional comorbidities.21 The appropriate diagnosis and management of UGIB has evolved significantly over the past decades; however, challenges still remain regarding risk stratification of patients with UGIB, the optimal timing of endoscopy, and delayed endoscopy.22-26
In cases of suspected UGIB, early risk stratification has proven beneficial in clinical decision-making regarding endoscopy timing, level of care required, and discharge planning. Currently, the risk stratification of patients with UGIB relies on clinical parameters and scoring systems that often require subjective interpretation by clinicians. The Glasgow-Blatchford scale (GBS), which incorporates both clinical and laboratory components, is implemented in current clinical guidelines to predict the risk and need for intervention.27 However, this scoring system is not consistently or universally used in patients presenting with UGIB symptoms. For example, Schembre et al.28 reported that of 644 patients who presented to the emergency department with clinical suspicion of UGIB, 37% were not risk-scored. Furthermore, several studies have suggested distinct interpretations of risk scores and current GBS cut-off values, as stated in the guidelines.29–33 Currently, comprehensive intake history, clinical symptoms, and pre-endoscopic risk scores are not sufficiently predictive for a definitive diagnosis. As a result, nearly every patient with suspected bleeding will have to undergo invasive diagnostic endoscopy with general anesthesia as a conclusive means to diagnose bleeding.34 Additionally, since UGIB and lower gastrointestinal bleeding (LGIB) require discrete clinical diagnostic pathways and symptoms at initial presentation do not always elucidate the anatomical source of bleeding, this can present an additional potential diagnostic challenge.35
Beyond risk scoring, the current clinical methods used to detect gastrointestinal bleeding include monitoring vital signs to indicate hemorrhage, taking a comprehensive history, paying attention to the clinical presentation (melena, hematemesis) and laboratory findings, and evaluating the current medication regimen of the patient.36 However, these methodologies can be imprecise and often require subjective clinical interpretation.37,38 Furthermore, UGIB is often acute in onset, and the available diagnostic methods do not allow for the indication of bleeding events in real time; instead, they only draw attention to potential hemorrhage after significant blood loss has already occurred.39 Hence, there is a need for more effective early identification of bleeding or rebleeding occurrences as an essential component to improve patient outcomes and potentially reduce the incidence of these emergent hemodynamic events.
A single-use ingestible capsule (PillSense System; EnteraSense) was developed to detect blood in patients presenting with suspected UGIB. This noninvasive device is intended to detect blood in the stomach in real time, providing direct evidence of UGIB and supplementing the current standard of care. The aim of this preclinical study was to assess the safety and performance characteristics of the PillSense System in a simulated bleeding model.
METHODS
This preclinical study used a porcine model (14 animals). Two subjects were acute studies, and 12 subjects survived for 2 weeks. The primary endpoints of the study were safety and sensitivity of blood detection. The secondary endpoint was the confirmation of device excretion.
Bleeding sensor device
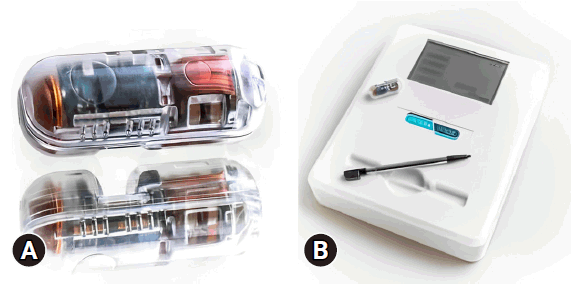
The PillSense System is a noninvasive and easy-to-use system designed to detect blood in real time. The overall system consisted of an optical-based sensor contained within an ingestible, pill-shaped capsule (11 mm×27 mm dimensions, similar to a capsule endoscope) (Fig. 1A) and a PillSense receiver (Fig. 1B), which is an external real-time display monitor. The capsule is a single-patient device that contains an optical-based sensor designed to detect blood during its natural propulsion through the digestive tract. Each wavelength is absorbed in a unique manner by the capsule, which allows the system to analyze each distinct light that is absorbed and identify those corresponding to the blood. Previous capsule testing confirmed that the device could discriminate between blood and food, including red-colored foods. The purpose of the capsule is to aid in the detection of UGIB without the use of photography. The capsule is designed to withstand the mechanical forces and chemical environment of the digestive system, passing its way through the gastrointestinal tract to be excreted naturally and painlessly from the body. The capsule itself is similar in terms of form to capsule endoscopy, which is typically excreted within 2 weeks and most often within 24 to 48 hours.40,41 A measurement data point was taken every 2 seconds by the sensor and wirelessly transmitted to the external receiver. The receiver plots the sensor output values in real time, generating a continuous chart during data acquisition alongside a numeric value ranging from 0 to 5, with an assigned threshold value of 1.8. Sensor output values above 1.8 indicate the presence of blood, while values below 1.8 indicate the absence of blood. In conjunction with the sensor output values, warning signals and intuitive “Blood detected” or “No blood detected” messages are displayed.
Study protocol
The bleeding sensor system was evaluated in an in vivo porcine model. The study population consisted of 14 female pigs (weight, 50–60 kg). The first two subjects were involved in acute studies with the objectives of assessing device functionality and defining the minimum blood detection threshold. The remaining 12 subjects underwent a 2-week survival study to confirm minimum blood detection, wireless communication, and eventual spontaneous passage of the device.
All subjects fasted for 12 hours prior to the procedure. Each subject was sedated with an injection containing ketamine (20 mg/kg) and xylazine (1 mg/kg), administered intramuscularly. Each animal was then intubated and administered inhalant isoflurane 2.5% to 4% for induction and isoflurane 0.5% to 4% for maintenance, delivered through a precision vaporizer. Subjects were placed in the left lateral decubitus position.
1) Acute study protocol (2 subjects)
The acute study protocol involved providing two subjects with the PillSense System intervention to acutely evaluate the sensor’s capabilities at different concentrations of blood. In both subjects, the endoscope (GIF-180; Olympus) was advanced into the stomach and all gastric fluid was suctioned out. Blood was drawn from the femoral vein of each swine and heparinized. Gastric fluid (50 mL) was re-instilled into the stomach, followed by instillation of 50 mL of water to simulate the expected ingestion of the capsule by the patient with a sip of water. Subsequently, the bleeding sensor device was paired with the receiver unit and the serial number of each device was recorded. The bleeding sensor device was then delivered endoscopically into the stomach. The endoscope light source was turned off before starting the measurement process.
After the capsule detected “No blood,” 5 mL of heparinized blood was instilled into the pool of gastric fluid followed by 5 mL of water. The addition of 5 mL of blood and 5 mL of water was repeated two more times, and ultimately, 20 mL of blood was added, followed by 5 mL of water. Sensor readings were recorded at blood concentrations of 0%, 4.5% (5 mL in 110 mL), 8.3% (10 mL in 120 mL), 11.5% (15 mL in 130 mL), and 22.5% (35 mL blood in 155 mL). Subsequently, the animals were euthanized.
2) Survival study protocol (12 subjects)
The survival study protocol entailed providing 12 subjects with the PillSense System intervention and monitoring them over a period during the entire capsule passage and excretion. The acute study protocol was repeated for 12 subjects. However, instead of increasing the concentration of blood, only 20 mL of heparinized blood was instilled into the stomach (which already contained 50 mL of gastric fluid and 150 mL of water). Therefore, only blood with 9% concentration (20 mL blood/220 mL) was tested. Additionally, the swine recovered, and fluoroscopy was performed every 5 to 7 days to monitor the location of the capsule until excretion was documented.
Statistical analysis
All continuous variables are reported as mean±standard deviation for normally distributed data. The paired Student t-test was used to compare continuous variables. Calculations were performed using the SAS ver. 9.4 (SAS Institute).
Ethical statement
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS), and Animal Welfare Act. The institutional animal care and use committee (IACUC) reviewed and approved the protocol (No. DB-631).
RESULTS
All 14 (100%) sensors performed well. Additionally, all 14 (100%) sensors detected “No blood” in the absence of blood (baseline reading).
In the acute study, the sensor output value increased from approximately one in the absence of blood to approximately three when the second 5 mL of blood was added (8.3%). The sensor output continued to maintain a high value of three at the higher concentrations tested (up to 22.5%). Figure 2 demonstrates the increase in the sensor output as a function of increasing blood concentration.

Blood detection results in the acute study. The graph demonstrates increasing PillSense System sensor output as a function of increasing blood concentration in the stomach.
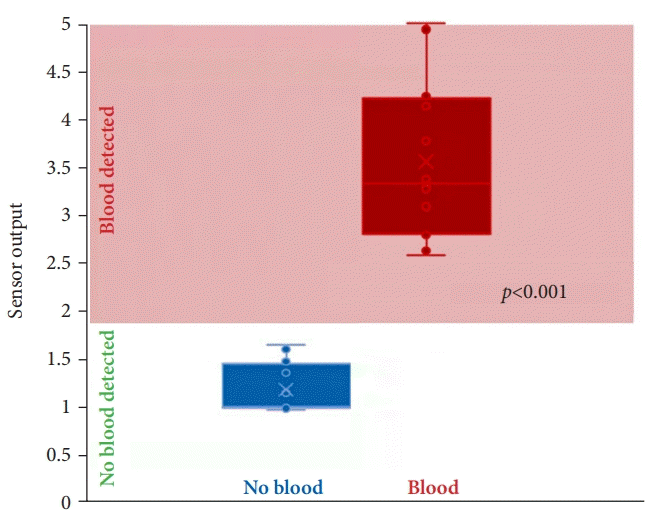
In the survival study, 12 of the 12 (100%) sensors detected a minimum blood concentration of 9% (20 mL whole blood in 220 mL total fluid). There was a significant difference between the mean sensor output in the absence of blood (1.18±0.26) and the mean sensor output at 9% blood concentration (3.56±0.86, p<0.001), as shown in Figure 3. There were no issues with the wireless connectivity or data streaming. Twelve of the 12 (100%) subjects survived for 2 weeks without concern for gastrointestinal blockage, and all devices in these 12 animals were spontaneously excreted.
DISCUSSION
UGIB is a potentially life-threatening condition that requires timely endoscopic evaluation and clinical intervention.42 This study aimed to detect and monitor UGIB using a novel ingestible capsule with a telemetric sensor for blood detection, the PillSense System, in an in vivo porcine model. Knowledge of the presence or absence of blood in the upper gastrointestinal tract can aid clinicians in evaluating the differential diagnoses regarding the localization of gastrointestinal bleeding. Since patients diagnosed with LGIB undergo an entirely different diagnostic workup than those diagnosed with UGIB,5 this information is an important parameter that can guide clinical decision-making processes, cost of care, and resource utilization. Currently, esophagogastroduodenoscopy (EGD) is the gold standard for both the diagnosis and treatment of acute UGIB. There is a paucity of reliable noninvasive diagnostic tools applicable to gastrointestinal bleeding, leaving the indication for emergency endoscopy a decision based on clinical observation. Thus, novel diagnostic tools that can accurately and safely determine the presence or absence of severe bleeding may aid in urgent triage and save resources by avoiding unnecessary emergency endoscopies and moving diagnosis away from subjective clinical decision-making.
The ability to accurately detect and monitor the presence of blood in the gastrointestinal tract is an essential component of risk stratification and clinical decision-making regarding the safe identification and assignment of patients to either emergency endoscopy or outpatient management. Hence, many researchers have turned to the use of endoscopic capsules in an effort to investigate the potential streamlining and improvement of risk stratification in patients with clinically suspected gastrointestinal bleeding.43 In particular, video capsule endoscopies (VCEs) have proven to be highly efficacious in their capability to detect and localize bleeding, as shown in several prospective clinical trials.44-48 Interestingly, Brunk et al.44 showed that by using VCEs, nearly 80% of hospital admissions made based on the GBS score alone could have been avoided. In another study, Chandran et al.45 demonstrated that five times more patients could be safely managed in an outpatient environment than currently managed using current GBS score cutoffs. Hence, previous studies have shown that risk stratification is possible and has potentially improved through the utilization of capsule technologies for clinical decision-making.
Along these lines, GBS scores have been shown to have controversial performance in patient groups considered to be very low-risk or high-risk.49 For example, a recent study by Lau23 showed inaccuracies in the ability of the GBS scoring system to appropriately stratify high-risk patients for either emergency EGD or elective EGD. However, current guidelines for low-risk patients state that a GBS score of 0 allows for safe discharge with subsequent outpatient management.29 However, more recent data have suggested that low-risk GBS groups could be further stratified, with the threshold expanded to a GBS score value between 1 and 3 to facilitate safe patient discharge and outpatient management.50,51 While doing so could potentially reduce costs, length of stay, and the pressure for urgent endoscopies, objective decisions for cutoffs have been difficult because appropriate interventions currently rely on physician interpretation of the GBS score. However, accurate risk stratification has shown immense value in terms of its ability to distinguish high-risk patients needing clinical intervention and hospitalization from lower-risk patients possessing a lower likelihood of developing complications and those who may be safely managed in outpatient settings.52,53 Previous studies have shown that this could reduce the number of hospital admissions by up to 20%.32 This study demonstrated that the PillSense System could safely and accurately detect gastrointestinal bleeding across a wide range of blood concentrations. Therefore, it may provide valuable and objective information for patients on the cusp of both the low- and high-risk categories and aid physicians in making more accurate risk stratifications to guide optimal, quick, safe, and cost-effective diagnoses in the case of UGIB.
Furthermore, in contrast to VCEs, the PillSense System is a non-imaging device that rapidly provides a dichotomous datapoint—either “Blood detected” or “No blood detected.” Hence, the interpretation of device results is straightforward and quick. In addition, as a simple ingestible capsule, the PillSense System can be successfully administered by nearly any type of care provider with quick training. In the present study, the blood concentration in the stomach of porcine models was detected at a minimum of 9% and increasing concentrations of up to 22.5%. Hence, this system offers real-time detection that may be beneficial in terms of time-to-treatment for patients who may require second-look endoscopies and early intervention for those with nonspecific or late-presenting clinical symptoms. This also means that the optic sensor can detect the presence of blood in diluted gastrointestinal fluids, providing superiority in terms of distinguishing between bleeding and non-bleeding stomach contents, where the human eye cannot easily communicate through endoscopic images such as those utilized by VCEs. Overall, this is particularly notable, as VCEs are significantly more time-consuming, and similar to EGD performance, they require more specialized staff and training, making them costly.54 More specifically, the standard EGD, for example, has been previously reported to cost between 2,000 USD and 3,000 USD.55 By contrast, capsule technologies currently in market are typically in the range of 250–700 USD, without factoring in additional costs associated with personnel and results interpretation, which the PillSense System will not require.41,56
Notably, none of the porcine models in this study with negative capsule results were found to have evidence of bleeding during further endoscopic workup. This is especially important because the confirmation of the absence of clinically relevant bleeding that would currently require endoscopic diagnostic evaluation within 24 hours has a large-scale value, potentially saving human and financial resources. Moreover, this study shows that the PillSense System allows for rapid and reliable interpretation of results, suitable for emergency settings, and warrants further investigation in prospective human trials. Furthermore, current technology cannot discern the amount of bleeding or distinguish the time or exact origin of the bleeding, all of which can serve as important indicators of severity and inspiration for future device iterations, which could expand the impact and application of the PillSense System in clinical use.
A primary limitation is that this was an acute animal study. Porcine models are established and evidenced large animal models for this type of study because the anatomy of these animals’ gastrointestinal tract is the most comparable to the digestive tract of humans, and the tubular organs allow for endoscopic procedures to be performed under clinically realistic circumstances.57 However, it is important to note that there are still anatomical differences between the upper gastrointestinal tracts of porcine and human models.58 Furthermore, the exact diagnostic accuracy in terms of sensitivity and specificity of the device remains to be clarified in in vivo models with randomized experimental conditions.
In conclusion, this study demonstrated the capability of an ingestible wireless sensor to detect and monitor gastrointestinal bleeding in real time and across a wide range of blood concentrations in an in vivo porcine model. The capsule could detect a small volume of fresh blood with successful wireless transmission of data to an external sensor and safe passage through the digestive system. Notably, none of the animals in this study with negative capsule results were found to have evidence of bleeding during further endoscopic workup. This is especially important because the confirmation of the absence of clinically relevant bleeding that would currently require endoscopic diagnostic evaluation within 24 hours has a large-scale value, potentially saving human and financial resources. Moreover, this study shows that the PillSense System allows for rapid and reliable interpretation of results, is suitable for emergency settings, and warrants further investigation in prospective human trials.
Notes
Conflicts of Interest
Kimberly F. Schuster has no potential conflicts of interest. Dr. Christopher C. Thompson and Dr. Marvin Ryou are paid consultants and have financial equity at EnteraSense, Inc.
Funding
This work was funded by EnteraSense Inc.
Acknowledgments
The authors thank Chiara Di Carlo for her critical review of the manuscript and assistance with figures.
Author Contributions
Conceptualization: CCT, MR; Data curation: CCT, MR; Formal analysis: KFS, MR, CCT; Investigation: CCT, MR; Methodology: CCT, MR; Project administration: CCT, MR; Supervision: MR; Validation: CCT, MR; Writing–original draft: KFS, MR; Writing–review & editing: KFS, MR, CCT.