Endoscopic treatment of upper gastrointestinal postsurgical leaks: a narrative review
Article information
Abstract
Upper gastrointestinal postsurgical leaks are life-threatening conditions with high mortality rates and are one of the most feared complications of surgery. Leaks are challenging to manage and often require radiological, endoscopic, or surgical intervention. Steady advancements in interventional endoscopy in recent decades have allowed the development of new endoscopic devices and techniques that provide a more effective and minimally invasive therapeutic option compared to surgery. Since there is no consensus regarding the most appropriate therapeutic approach for managing postsurgical leaks, this review aimed to summarize the best available current data. Our discussion specifically focuses on leak diagnosis, treatment aims, comparative endoscopic technique outcomes, and combined multimodality approach efficacy.
INTRODUCTION
Leaks, perforations, and fistulas, though often used interchangeably, are different types of transmural defects and are associated with different endoscopic closure rates.1 Most of the literature so far has evaluated the efficacy of endoscopic therapy for transmural defects in general rather than for leaks alone.2
Leaks are defined as abnormal communications between the intraluminal and extraluminal compartments, usually owing to a defect in the integrity of the gastrointestinal wall. Upper gastrointestinal (UGI) postsurgical leaks (PSL) have increased in prevalence in recent years3 and are the strongest independent risk factor for postoperative mortality.4
Management of PSL is often challenging and may require radiological, endoscopic, or surgical intervention.5 Traditionally, either rescue surgery or a watch-and-wait strategy followed by surgery if symptoms persist have been the preferred therapeutic approaches. Recently, endoscopy has been emerging as a first-line therapeutic approach and is associated with lower morbidity and better quality of life compared to surgery.6 Steady advancements in interventional endoscopy in recent decades have allowed the development of new endoscopic devices and techniques that provide a more minimally invasive and effective therapeutic option for PSL than surgery. There are multiple endoscopic surgical options available which can be used as solo therapy or in combination with other surgical techniques.
Currently, there is no consensus regarding the most appropriate therapeutic approach for the management of PSL. Due to the continued widespread use of a watch-and-wait strategy in clinically stable patients, leaks are often referred late for endoscopic treatment. Late referral is unfortunately associated with worse endoscopic outcomes.7 Even when diagnosed early, endoscopic management remains complex and often requires multiple endoscopic treatments spanning several months. In this review, we aimed to summarize the best available data on the treatment of UGI with PSL.
POSTSURGICAL LEAKS
Leaks may occur immediately after surgery or, more commonly, after several weeks. Acute leaks are commonly attributed to technical issues such as anastomotic tension, stapler malfunction, or suture or staple line seepage. More delayed leaks can result from poor healing, usually due to ischemia at the staple-line or anastomosis.8,9 Several risk factors for leaks have been identified, including age, male gender, need for emergency surgery, smoking, alcohol abuse, American Society of Anaesthesiologists score, body mass index (BMI) >30 kg/m2, BMI <18.5 kg/m2, malnutrition (albumin <3 g/dL), prolonged operative time, anemia, intraoperative blood loss, diabetes, hypertension, renal failure, cardiovascular disease, steroid use, or atherosclerotic calcification of the aorta and the arteries supplying the gastric tube.10-12 Identifying preoperative risk factors can raise clinical suspicion for early leak diagnosis.
The clinical presentation can range from asymptomatic leaks (diagnosed on incidental imaging) to sepsis-related multi-organ failure. Common initial clinical signs include fever and intra-thoracic or intra-abdominal abscesses.10 Chronic leaks have a more insidious presentation. Inspection of surgical drains (if present) helps in the early identification of a surgical leak.13 Although fluoroscopy with a water-soluble contrast medium and computed tomography (CT) with oral contrast are the best imaging modalities for diagnosis, they are prone to false-negative results. CT scan findings include free or contained extraluminal gas, fluid, or contrast material in the mediastinum or abdomen, or visualization of a transmural defect.14 In addition, CT scans allow inspection of regions beyond the esophagogastric lumen. Endoscopy is a reliable diagnostic modality,15 although its diagnostic value seems to be lower for cervical anastomotic leaks.16 Endoscopic examination is crucial to help identify leaks in uncertain cases and to obtain additional critical information such as the extent of tissue disruption, loss of tissue viability, and the presence of downstream strictures that may perpetuate the leak.2,15 The combination of CT and endoscopy is emerging as the gold standard to diagnose PSL as both mucosal integrity and perianastomotic conditions can be examined.10
PSL management is based on several factors, the most important of which include patient stability and time from surgery.5 Spontaneous closure with conservative and radiological interventions is highly variable, with reported rates ranging from 16% to 46%.17,18 Complex and larger leaks are unlikely to heal spontaneously. Factors that predispose patients to delayed or absent spontaneous leak closure include older age (>65 years), malnutrition, high-output drainage, associated malignancy, previous radiation therapy, immunosuppression, sepsis, diabetes, renal failure, and chemotherapy.19,20
AIMS OF TREATMENT
The aim of PSL therapy is to reestablish digestive tract continuity, prevent or treat infections, reduce risk of further contamination, drain fluid collections, and provide nutritional support.21 Determining optimal therapy requires the careful examination of a patient’s clinical status, leak characteristics (site, length, time, and presence of necrosis), and a review of all available technical options and surgical expertise.
SURGICAL TREATMENT
The choice of surgical option for PSL depends mostly on the leakage site and the presence or absence of necrosis. It is usually limited to patients with severe sepsis, with an uncontained leak (allowing irrigation and drainage of intra-abdominal collections), with defects not amenable to endoscopic closure, or after failed endoscopic treatment.3,21 Reported outcomes of salvage surgical procedures is often prone to selection bias, as patients are generally sicker or have failed multiple previous therapies. Despite the high morbidity and mortality of salvage surgical therapy,22 it should not be ignored if deemed appropriate, for fear of complications or a poor outcome.
ENDOSCOPIC TREATMENT AND OUTCOMES
The available endoscopic approaches range from primary and secondary closure techniques using endoluminal suturing devices, over-the-scope clips (OTSCs), fibrin glue, diversion with stents, endoscopic internal drainage (EID) using nasocystic drains or double-pigtail stents, endoscopic vacuum therapy (EVT), and septotomy with or without pneumatic dilation.
A summary of the best available evidence per technique and between techniques is summarized in Tables 1 and 2, respectively.23-37
Evaluation per technique
1) Stents
Stents are the endoscopic treatment of choice for oncologic and bariatric leaks, with ample supporting evidence (Fig. 1). Reported clinical success rates range from 48% to 100%.38-42 Multiple endoscopic sessions using multiple stents, as well as use of other adjunctive therapies, may be necessary to achieve leak closure.38,39,43,44 Van Halsema and van Hooft45 reported a clinical success rate of 81.4% for PSL in a 247-patient cohort. Based on three systematic reviews of endoscopic stents,23-25 clinical success in oncologic leaks and perforations ranged from 81% to 87%, with no significant differences among stent types. Repeat endoscopic intervention was needed in 17% to 25% of patients, and 7% to 13% required further surgical intervention. Median stent indwell time ranged from 5 to 10 weeks.45 Even though clinical success rates between stents are comparable, self-expandable metal stent (SEMS) have been reported to have higher technical success rates, reduced risk of migration and subsequent need for stent repositioning, as well as lower risk of perforation compared to self-expandable plastic stents.24 Regarding bariatric leaks, leak closure and adverse event rates range from 65% to 100% and 14% to 86%, respectively, with migration being the most frequent adverse event reported with rates of 5% to 67%.38-42 A recent meta-analysis26 reported an 89% leak closure rate with stent migration occurring in 23% of cases, with the higher success rate possibly due to the more frequent use of stents designed to treat post-bariatric leaks. Recent reports using bariatric stents have shown similar success rates without statistically significant differences in migration rates when compared with conventional stents,40,46,47 with a potentially higher risk of perforation and chest pain.48

Endoscopic image of a post total gastrectomy leak (A) with an associated collection (B), with surgical drain in place. A fully covered self-expandable metal stent (28/23/28×155 mm) was placed covering the leak (C). Stent was removed 40 days later with leak resolution (D).
Delayed stent placement,49 persistent leakage after initial stent placement,50 proximal esophageal leaks, stents traversing the gastroesophageal junction, larger leak defects, and distal conduit leaks51 are associated with a higher chance of oncologic leak treatment failure. A recent study suggested that stents should not be used for leaks extending more than 30% of the luminal circumference.3 Leaks larger than 1 cm52 and delayed stenting can also affect endoscopic outcomes in bariatric leaks.38,53,54
Though SEMS complications are fairly common (20% to 72%),7 most are usually mild and can be managed conservatively. Stent migration is the most common adverse event, however, in rare cases, severe bleeding and perforation may occur.23-25,40,53 Migration rates are higher with fully covered (FC) SEMS than with partially covered SEMS.55 However, fixation techniques such as OTSCs or suturing may reduce its occurrence56,57 without the difficulties associated with PC-SEMS removal.58 Other methods to reduce the risk of stent migration include the Shim technique59 as well as using wider diameter stents.55,60
2) Over-the-scope-clips
Several studies have reported on the effectiveness and safety of OTSCs with most focusing on all types of transmural defects. Haito-Chavez et al.61 reported a 73% success rate in 30 anastomotic leak cases. In contrast, Baron et al.62 and Honegger et al.63 reported success rates below 33% for post-esophagectomy leaks, potentially due to the relatively narrow diameter of the esophageal lumen. A recent systematic review of 1,517 cases found a 66% OTSC success rate in a subset of 97 anastomotic leak cases.27 Another systematic review of anastomotic leaks reported a 73% clinical success rate.28 In the context of post-bariatric leaks, a closure rate of 67.1% was reported.26 The number of endoscopic sessions ranged from 2 to 7.64
Clinical success has been reported to be higher when OTSCs are applied as a primary therapy (69.1% vs. 46.9%),61 within one week of diagnosis, and in defects with minimal inflammation and fibrosis.65,66 Leaks larger than 13 mm are also associated with increasing failure rates.65
3) Suture
The largest study evaluating endoscopic sutures included 122 patients, of which 15 had anastomotic leaks, with a clinical success rate of 27% in leak closure.29 The tissue status and suture feasibility of the wall defect layers were the main outcome predictor. A case series of full-thickness endoscopic suturing of post-sleeve gastrectomy leaks suggested that suturing alone may be sufficient to treat small acute leaks; however, larger leaks would likely require adjunctive therapies such as SEMS placement.67
4) Tissue sealants
The reported success rate of tissue sealants is highly variable, ranging from 55.7% to 96.8%,68-70 with complication rates reaching 12.5%.26 The efficacy of glue sealants as a primary treatment of PSL is controversial71 since they are usually adjunctive to other primary treatments such as stents and clips.69,72 Reported outcomes are difficult to interpret and prone to bias. Sealants might be more suitable for small leaks (<15 mm), leaks without concurrent infection,73 or residual small collections after the use of other techniques.72 Complete leak closure might require the adjunctive use of vicryl plugs or multiple sealant applications (one to nine sessions repeated every two to three days).26,68
5) Endoscopic vacuum therapy
EVT is typically performed using polyurethane sponges (Fig. 2). Macroporous low-density sponges are commonly used because of their greater debriding capacity and stronger contraction under negative pressure, which leads to a more pronounced wound cavity shrinkage (macro-deformation). Permeable films have significant advantages as connection materials compared to polyurethane foam-based drains depending on the clinical indication. These “open-pore film drains,” in which the perforated area of the drain is directly wrapped with an open-pored film, are easier to place due to their smaller diameter and are less adherent to the wound cavity, allowing easier removal.74
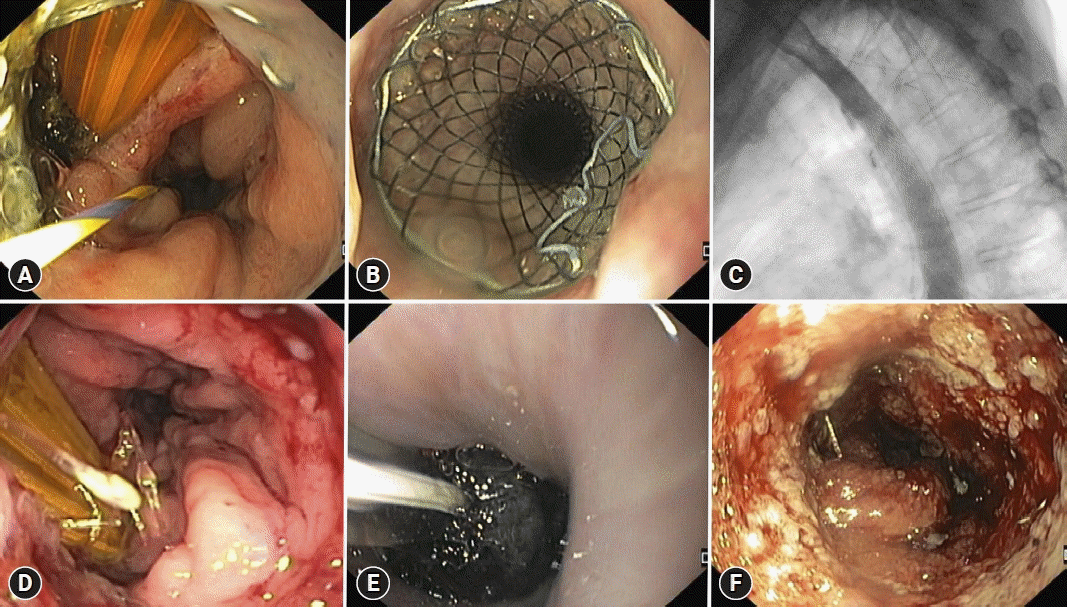
Endoscopic image of a post Mckeown esophagectomy leak, with a surgical drain in place and a guidewire placed in the gastric lumen (A). A partially covered self-expandable metal stent (28/23/28×155 mm) was placed covering the leak (B, C), however, leak persisted after stent removal (D). Intraluminal endoscopic vacuum therapy was performed (E) with leak resolution after two sponge exchanges (F).
The reported clinical success rate of EVT varies widely, ranging from 66.7% to 100%. Schorsch et al.75 and Laukoetter et al.76 reported leak closure rates of 95.2% and 92.3% in 21 and 39 patients, respectively. Median treatment durations were 11 (range, 4–46) and 20 days (range, 3–104), respectively. Bludau et al.77 reported a healing rate of 77.9% in a cohort of 59 patients. In several studies, additional therapies such as OTSCs or stents were placed after EVT. A recent systematic review of oncologic leaks reported 79.5% and 90% clinical success rates for esophagectomy and gastrectomy leaks, respectively, with stenosis rates reaching 15.9% and 9.2%, respectively.30 Neoadjuvant treatment, rescue application, and intraluminal leak location have all been associated with a higher risk of EVT failure.78
Adverse event rates range from 4.1% to 12.0%, with the majority being minor such as limited bleeding upon sponge exchange, sponge dislodgement, discomfort from repeated procedures, or stricture formation after EVT therapy.73,79 Rarely, major events like significant bleeding can occur.76,80,81
A recently developed technique combining EVT with luminal stenting (VACStent; VAC Stent GmbH) allows oral enteral feeding, continuous drainage, and wound healing.82,83 This technique is only suitable for intraluminal EVT because of the cylindrical shape of the polyurethane foam. The available evidence for this new system is limited to small case series.84
6) Endoscopic internal drainage
The largest study evaluating EID (Fig. 3) as a first-line approach for sleeve gastrectomy leaks (n=617) reported an overall clinical success rate of 84.7%, median treatment duration of 80 days (interquartile range, 29–128 days), and a complication rate of 4.5%.31 Complications were managed conservatively in approximately half of the cases. Donatelli et al.85 reported the use of double pigtails as a first-line approach in 67 patients achieving a 78% leak closure rate. Bouchard et al.86 reported EID outcomes in 33 patients post-sleeve gastrectomy or gastric bypass with persistent fluid collections (despite previous endoscopic treatment in 19 patients), with a 78.8% clinical success rate after a mean of 115 days (range, 23–773). Gonzalez et al.87 reported the outcomes in 44 patients with sleeve gastrectomy leaks, either as first-line treatment (n=22) or after prior therapy (n=22). The efficacy was comparable between the groups (86% vs. 82%, respectively), with a median 3.0–6.0 vs. 4.5±2.4 number of endoscopic sessions. Healing time from endoscopy was 46 days excluding follow-up.87 EID for oncologic leaks has not been widely studied. Recently, Hallit et al.32 and Donatelli et al.88 reported success rates of 100% in 38 and five patients, respectively.

Endoscopic image of a post-sleeve gastrectomy leak (A, B), with an associated perigastric collection, visible on fluoroscopy (C). Endoscopic internal drainage of the collection was performed with placement of one double-pigtail plastic stent (7 Fr 4 cm) across the leak orifice (D), with drainage of purulent content (E). Fluoroscopic image of the double-pigtail stent, with one extremity in the perigastric collection and the other in the gastric tube (F).
Adverse events such as discomfort, ulceration, dysphagia, and splenic hematoma are rare.86 When combined with surgical cleansing in patients presenting with severe sepsis, EID allows early surgical drainage removal and a reduction in chronic fistula tract formation.89 Longer delays between diagnosis and treatment, larger leaks, sepsis, presence of gastrobronchial fistula, and previous OTSC deployment are risk factors for treatment failure.33
7) Endoscopic septotomy
Endoscopic septotomy may be used as a first-line or salvage therapy, with clinical success rates ranging from 70% to 85%.89-91 Baretta et al.92 reported their experience with endoscopic septotomy in 27 patients with post-bariatric leaks. After one to six endoscopic sessions, all patients achieved leak resolution with a mean healing time of 18 days. More than half of the patients underwent additional dilatation of the angularis incisura stenosis. Complications included perforation and bleeding.93
Comparison between techniques
There are a limited number of studies comparing efficacy of different endoscopic modalities for the management of leaks. Farnik et al.34 retrospectively compared FC-SEMS and OTSCs and reported leak closure rates of 69% and 31%, respectively; clinical success after primary intervention was 40% for FC-SEMS and 70% for OTSCs. However, defects treated with FC-SEMS were larger than those treated with OTSC (12.6 mm vs. 7.1 mm). Manta et al.94 primarily utilized OTSCs or combined OTSCs with SEMS, with leak closure rate of 81% to 85%. Lorenzo et al.33 reported better outcomes with EID than with a combination of stents, tissue sealants, and OTSCs (86% vs. 64%, p=0.55) in 100 patients with post-sleeve gastrectomy leaks.
Recently, the outcomes of SEMS placement were compared with those of EVT for PSL treatment in several meta-analyses. EVT was associated with a higher leak closure rate (16%–21% higher), a lower mortality rate (10%–12% lower),30,35,36 fewer adverse events,35 and shorter treatment duration,35,36 with no difference in the length of hospital stays.30,35 These results have not been replicated in all studies. Berlth et al.,95 in a large cohort of 111 patients with post-esophagectomy leaks, reported a closure rate of 85.7% for EVT vs. 72.4% for SEMS. This difference was not statistically significant.
Recently, EID has also been compared with stent placement and EVT. Hallit et al.32 reported higher closure rates with EID than with stent placement for the treatment of oncological PSL (95% vs. 67%, p=0.002). The success rate increased to 100% and 77% after using adjunctive therapies (OTSC and crossover to EID, respectively). In univariate analysis, only primary EID use was associated with treatment success. Jung et al.37 compared EID and low-negative pressure EVT for oncological PSL and reported better overall success rates (100% vs. 85.2%, p=0.03) and primary success rates (91.4% vs. 74.1%, p=0.09) with EID. EVT had a shorter treatment duration but required more sessions. However, the two cohorts of patients were not treated uniformly since each institution performed only one type of endoscopic treatment. Low negative pressure applied during EVT could have affected its efficacy.
Multimodality approach
Bège et al.96 assessed multimodal treatment in 27 patients who underwent bariatric PSL. Primary procedures were successful 41% of the time and all patients achieved leak resolution after a mean 4.4 endoscopic sessions and a mean of 86 days. Rodrigues-Pinto et al.55 performed the largest multicenter study, which included 206 patients treated with UGI PSL. Although high overall clinical success (80.1%) and leak resolution rates (83.5%) were achieved, the first endoscopic technique was successful in only 44% of leaks and multimodal therapy was often required (40.8% of the time). Clinical success correlated with the duration of treatment, with leak resolution rates reaching a plateau between the third and fourth endoscopic techniques (approximately 70–80%), and a median time to leak closure of 52 days. Only 10% of the leaks successfully closed after 125 days of treatment. A different study also demonstrated a reduced endoscopic resolution rate of sleeve gastrectomy leaks over time, from 76.4% in the first postoperative month to 48.5% after six months.97 This reflects the need to better define endoscopic failure. In a survey study, although there was no definitive definition consensus, persistent inflammation with clinical sepsis and the impossibility of resuming oral feeding were suggested as components of the definition.98 The inability to close the leak over time, especially after four months of treatment, should also prompt consideration of therapeutic alternatives such as surgery.
TREATMENT SELECTION
Despite the increasing effectiveness of EID and EVT in the treatment of PSL, stent placement remains the most widely available and frequently used technique in current practice.55,97,98 The approach to UGI PSL should always be tailored in a patient-specific manner. Leak location, size, chronicity, and associated cavities are the most relevant leak characteristics to consider when deciding the treatment.98 The type of previous surgery should also influence therapeutic decisions: EVT and stent placement (with or without percutaneous/surgical drainage) is a good option in oncologic leaks, whereas EVT and EID are best after bariatric surgery.98 Early referral of leaks is the most important predictor of treatment success.55,97,99,100
Considering most responses to a survey study,98 stent placement is commonly used for acute and small leaks without associated collections (defects up to 3 cm in size), OTSC placement for defects up to 1 cm in size, and endoscopic suturing for defects up to 2 cm in size. In the setting of an associated fluid collection, stents can be considered if external drainage is also performed. Otherwise, EVT and EID are options for acute and chronic leaks, whereas endoscopic septotomy can be performed for leaks lasting more than 4 weeks. Although endoscopic septotomy can be considered for all leak sizes, EVT is ideal for leaks > 2 cm in size.
CONCLUSIONS
Therapeutic endoscopy for UGI PSL management is safe, effective, and reproducible when a skilled endoscopy team is available. However, endoscopic management should be personalized and multidisciplinary, involving close collaboration among interventional endoscopists, radiologists, and surgeons. There is wide expert variation in the management of these patients, emphasizing the need to identify patients as early as possible and to select the best therapeutic option for each patient. Comparisons between different approaches are difficult because of heterogeneous study populations, the retrospective nature of relevant studies, lack of uniform definitions, and lack of prospective comparative studies. Therefore, it is difficult to establish a standard therapeutic algorithm. Combined treatment with simultaneous or sequential use of several endoscopic methods appears to be optimal for the management of UGI PSL. Future research should focus on assessing the effectiveness of combined therapies rather than focusing on individual endoscopic methods alone.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: ERP; Data curation: all authors; Formal analysis: all authors; Investigation: all authors; Methodology: all authors; Supervision: ERP; Validation: ERP; Visualization: ERP; Writing–original draft: all authors; Writing–review and editing: all authors.